Synthesis method of R-thiazolidine-2-thione-4-carboxylic acid
A technology of tetrahydrothiazole and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low preparation efficiency, affecting industrialized production, long processing time, etc., and achieve the effects of simple preparation conditions, improved preparation efficiency, and reduced synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
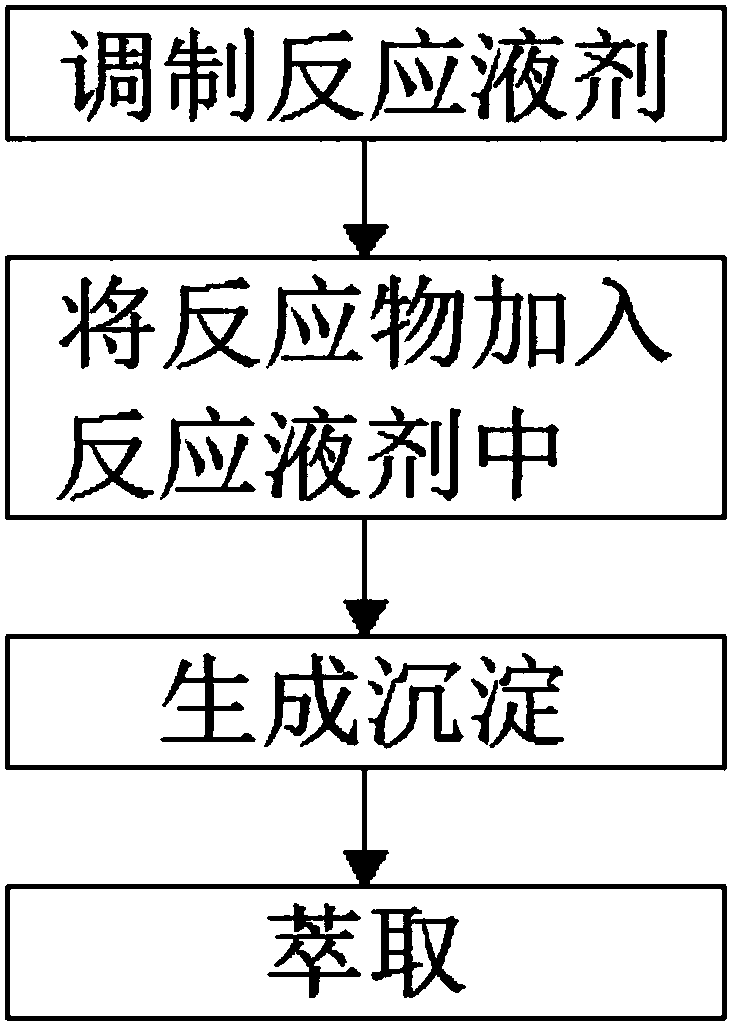
[0017] see figure 1 , the present invention provides a technical solution: a synthetic method of R-tetrahydrothiazole-2-thione-4-carboxylic acid, the synthetic method of R-tetrahydrothiazole-2-thione-4-carboxylic acid Concrete synthetic steps are as follows:
[0018] S1: Prepare the reaction solution: select a 400ml beaker, and add CuSO to the beaker 4 , ZnSO 4 、Al 2 (SO 4 ) 3 mixed solution, the CuSO 4 , ZnSO 4 、Al 2 (SO 4 ) 3 The volume of the mixed solution is 240ml, inject NAOH solution into the mixed solution, the concentration of the NAOH solution is (60-75)%, adjust the pH value to 12-12.5;
[0019] S2: Add reactants to the reaction solution: inject β-chloroalanine and CS into the reaction solution prepared in step S1 2 , the β-chloroalanine and CS 2 According to the molar ratio of 1: (1.0-1.2) specific gravity ratio;
[0020] S3: Generate precipitation: Stir the beaker filled with the reaction liquid in step S2, and stir in the same direction for 30-50 min...
Embodiment approach
[0024] (1) β-Chloroalanine and CS 2 According to the molar ratio of 1:1.0 proportion ratio;
[0025] (2) β-Chloroalanine and CS 2 According to the specific gravity ratio of 1:1.1 in molar ratio;
[0026] (3) β-Chloroalanine and CS 2 According to the molar ratio of 1:1.2 proportion ratio.
[0027] Implementation results: after the reaction of the first group of embodiments, there is more β-chloroalanine remaining; the second group of embodiments has a relatively moderate proportion of reactants, which saves raw materials; 2 There are more left.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com