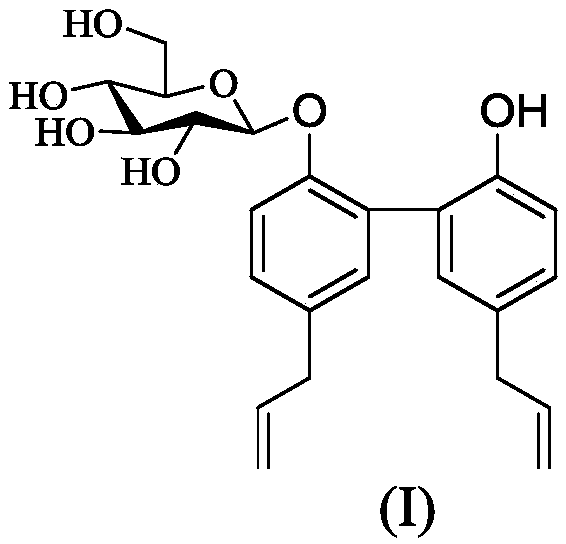
Application of magnolol glucoside to preparing central nervous system disease treating drugs
A technology of central nervous system and honokiol, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Compound 1:
[0026] ①Take 1.56g (4mmol) of 1,2,3,4,6-pentaacetylated glucose and dissolve it in 10.0mL of dichloromethane; add 1.72mL of acetic acid solution containing 30% HBr in an ice bath (10mmol), reacted for about 4 hours; after TLC detected that the reaction was complete, add 40mL dichloromethane to dilute, wash three times with 150mL ultrapure water (50mL each time), and the organic layer was washed with anhydrous Na 2 SO 4 Drying; the organic layer was recovered under reduced pressure to obtain the product 1-α-Br-2,3,4,6-tetraacetylated glucose (1.62 g), with a yield greater than 99%. ②Weigh 0.5mmol (133mg) of magnolol and dissolve it in 10mL of 0.8N NaOH aqueous solution; weigh 0.5mmol (206mg) of 1-α-Br-2,3,4,6-tetraacetylated glucose and 0.05mmol of tetrabutylammonium bromide (catalyst, TBAB), fully dissolved in 5.0mL of chloroform; the dissolved chloroform solution was added dropwise in the magnolol solution, and constantly stirred, reacted...
Embodiment 2
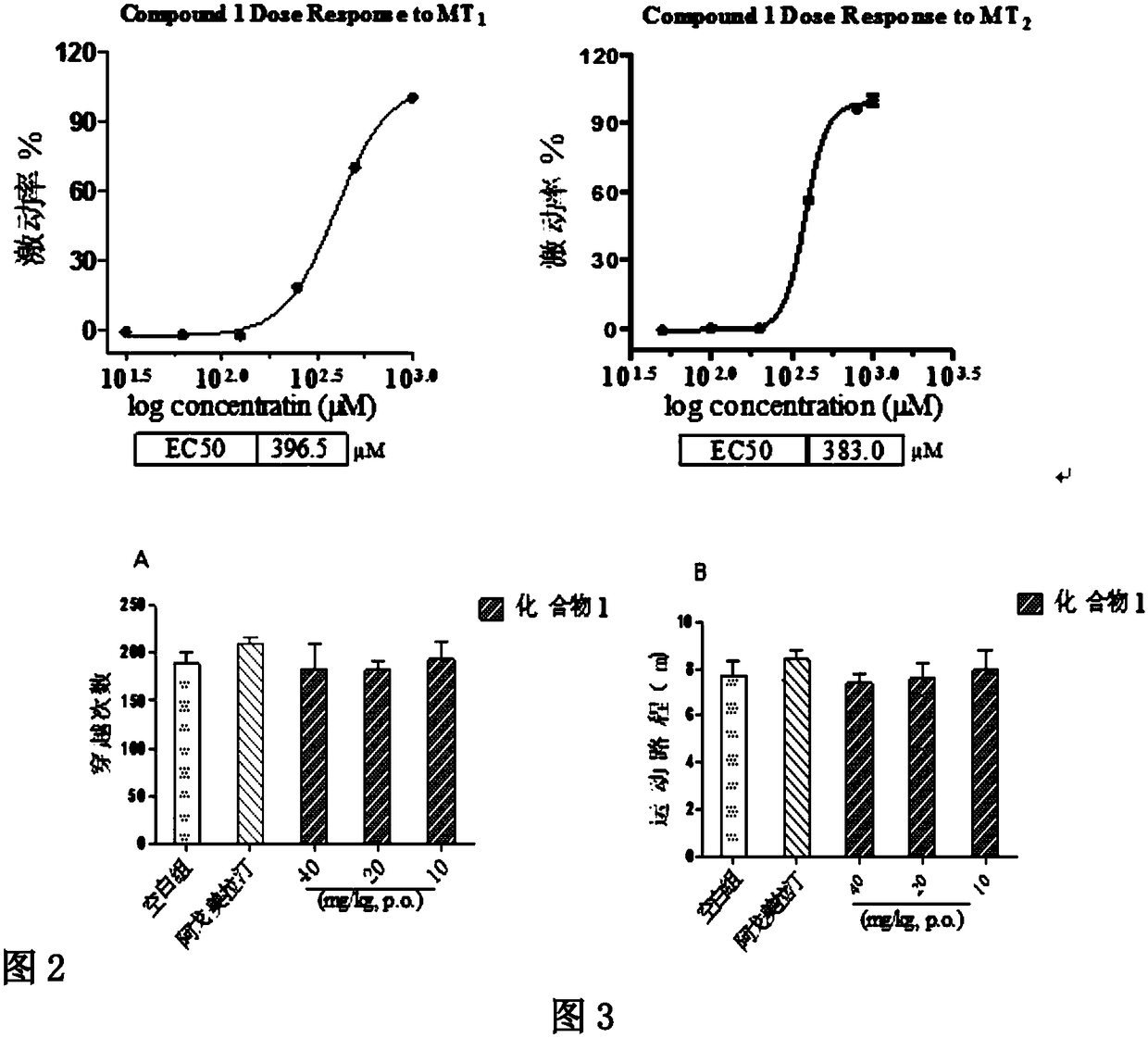
[0039] Compound 1 on melatonin receptor MT 1 and MT 2 Agonistic activity of the receptor.
[0040] 1 Materials and methods
[0041] 1.1 Materials:
[0042] melatonin receptor MT 1 and MT 2 The cell lines used for agonistic activity screening correspond to human kidney epithelial cells HEK293-MT 1 and HEK293-MT 2 ; Cell culture medium (Dulbecco's Modified Eagle Medium, DMEM) containing 10% fetal bovine serum; No-wash calcium flow kit.
[0043] 1.2 Instrument: CO 2 Constant temperature incubator Thermo Forma 3310 (USA); Inverted biological microscope XD-101 (Nanjing); Flexstation 3 Benchtop Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, California, USA).
[0044] 1.3 Experimental process
[0045] Coat the 96-well black-walled transparent-bottom cell culture plate with the substrate BD Matrigel, put it in a constant temperature incubator at 37°C for 1 hour, absorb the supernatant, and dilute it with 4×10 4 Density per well, the corresponding HEK293 cells we...
Embodiment 3
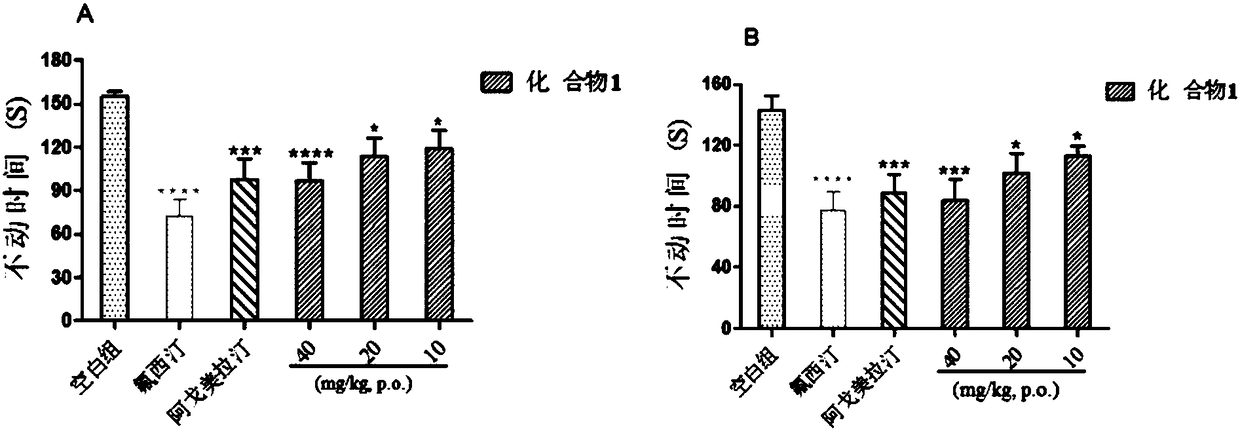
[0051] Effects of compound 1 on behavioral animal models related to depression
[0052] 1 Materials and methods
[0053] 1.1 Materials: Fluoxetine hydrochloride (Sarn Chemical Technology Co., Ltd., Shanghai, China); agomelatine (Sarn Chemical Technology Co., Ltd., Shanghai, China). Sodium carboxymethylcellulose 800-1200 (Sinopharm Group, Shanghai, China). Kunming mice 18-20 g (Beijing Huafukang Biotechnology Co., Ltd., Beijing, China), license number: SCXK (Beijing) 2014-0004.
[0054] 1.2 Method: The mice were divided into random groups, 10 in each group, and measured after 5 consecutive days of administration. Spontaneous activities mice were put into the bottom center of a black dark box with a length, width and height of 24x24x40cm, and the ANY-maze automatic collection system (Anymaze, Stoelting Co., Wooddale, USA) was used to record for 6 minutes, and the time was 5 minutes and 30 seconds after analysis. The number of crossings and the total distance traveled by the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com