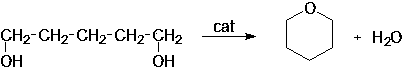Method for preparing tetrahydropyrane by dehydrating and cyclizing 1,5-pentanediol
A technology of dehydration cyclization and tetrahydropyran, applied in the direction of organic chemistry and the like, can solve the problems of few industrialized production methods and less tetrahydropyran, and achieve the effects of simple reaction steps, simple industrial scale-up and small technical investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation steps of the supported heteropolyacid salt catalyst are the same as those of the supported heteropolyacid catalyst, and will not be repeated here.
[0019] What the rectification in the tetrahydropyran preparation method of the present invention adopts is batch rectification, and can select continuous rectification in actual industrial production.
[0020] In the present invention, the main reaction formula for preparing tetrahydropyran by dehydration and cyclization of 1,5-pentanediol is as follows:
[0021]
Embodiment 1
[0023] In a flask equipped with a short fractionation column, add 500ml of 1,5-pentanediol and 15g copper phosphotungstopolyacid / activated carbon catalyst, and carry out dehydration and cyclization reaction under normal pressure, wherein the phosphotungstic heteropolyacid copper in the catalyst is The loading is 10 wt%. Control the reaction temperature to 170°C, control the top temperature of the fractionation column to 95°C, collect the distillate, and the reaction ends after 90 minutes. The distillate is purified by intermittent fractional distillation. Under normal pressure, light components such as water and by-products are removed first, and the distillate with a tower top temperature of 88±1°C is collected as tetrahydropyran. Others Heavier components with a higher boiling point than tetrahydropyran remain in the bottom of the column. The purity of tetrahydropyran is 98.3wt%, and the yield of tetrahydropyran is 83.1%.
Embodiment 2
[0025] In a flask equipped with a short fractionation column, add 200ml of 1,5-pentanediol and 6g of copper phosphotungstopolyacid / kaolin catalyst, and carry out dehydration and cyclization reaction under normal pressure, wherein the amount of copper phosphotungstic heteropolyacid in the catalyst is The loading was 25 wt%. Control the reaction temperature to 260°C, control the top temperature of the fractionation column to 90°C, collect the distillate, and the reaction ends in 30 minutes. The distillate is purified by intermittent fractional distillation. Under normal pressure, light components such as water and by-products are removed first, and the distillate with a tower top temperature of 88±1°C is collected as tetrahydropyran. Others Heavier components with a higher boiling point than tetrahydropyran remain in the bottom of the column. The purity of tetrahydropyran is 98.1wt%, and the yield of tetrahydropyran is 87.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com