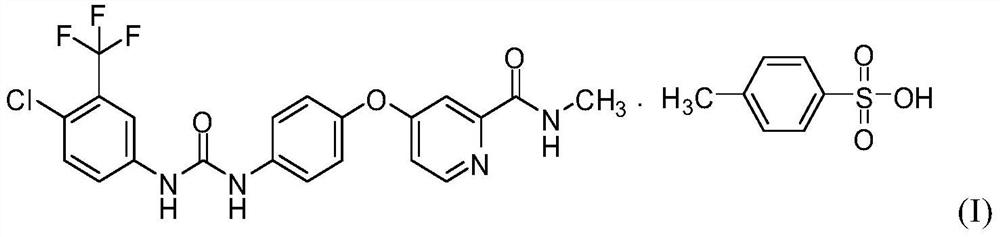
The preparation method of Sorafenib tosylate crystal form III
A technology of fennel crystal form and toluenesulfonic acid, which is applied in the field of medicinal chemistry, can solve the problems of large phase difference, easily exceeding the standard of dissolution residue, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of the present invention adopts the reverse drop technique, and the organic solvent of p-phenylmethanesulfonic acid and Sorafenib free base is added to crystallize in methanol, or the organic solvent of Sorafenib free base is added to p-phenylmethanesulfonic acid Crystallization in the methanol solution of acid can effectively control the residual solvent, and the small particle size of the product is conducive to drying into type III.
[0042] The main advantages of the present invention are:
[0043] 1. The preparation method of the present invention does not need to prepare other crystal forms in advance, the process cycle is short, and the operation is simple;
[0044] 2. The residual amount of organic solvents such as NMP, DMSO, DMF and the like in the crystal form III obtained by the preparation method of the present invention is low;
[0045] 3. The methanolate obtained by the preparation method of the present invention has a small particl...
Embodiment 1
[0052] Example 1: Preparation of Form III-1
[0053] Add 10 g of sorafenib free base and 6 g of p-benzenemethanesulfonic acid into 30 mL of N-N-dimethylformamide, stir and dissolve at room temperature, and filter to obtain filtrate A.
[0054] Take 180mL of methanol in a 500mL three-necked flask, and cool it to an internal temperature of 0-5°C with mechanical stirring in an ice-water bath. At this time, slowly add filtrate A to precipitate solids. After the addition of filtrate A is completed, keep stirring at 0-5°C for 1 hour, The resulting solid A was collected by filtration.
[0055] The obtained solid A was then added to 200 mL of methanol, slurried at room temperature for 1 h, and finally the obtained solid B was collected by filtration, and solid B was dried at 80° C. to obtain 12.45 g of crystal form III-1 with a molar yield of 91%.
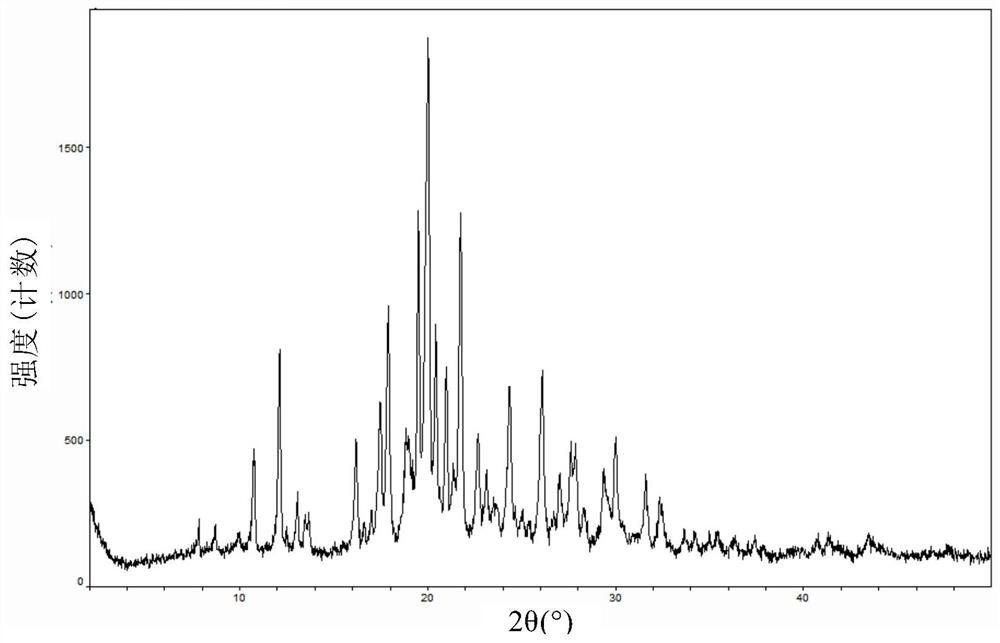
[0056] After determination and analysis, the obtained crystal form III-1 has the following properties: figure 1 The XRD pattern shown. ...
Embodiment 2
[0057] Example 2: Preparation of Form III-2
[0058] Add 10 g of sorafenib free base to 30 mL of N-N-dimethylformamide, stir to dissolve at room temperature, filter, and obtain the filtrate as A.
[0059] Weigh 6g of p-phenylmethanesulfonic acid and dissolve it in 180mL of methanol, and use mechanical stirring to cool in an ice-water bath to an internal temperature of 0-5°C. At this time, slowly add filtrate A to precipitate solids, and keep warm at 0-5°C after the addition of filtrate A is completed. After stirring for 1 h, the resulting solid A was collected by filtration.
[0060] The obtained solid A was then added to 200 mL of methanol, slurried at room temperature for 1 h, and finally the obtained solid B was collected by filtration, and solid B was dried at 80° C. to obtain 12.45 g of crystal form III-2 with a molar yield of 91%.
[0061] After determination and analysis, the obtained crystal form III-2 has basically the following figure 1 The XRD spectrum shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com