Energy-saving environment-friendly continuous preparation method of iohexol
An energy-saving and environment-friendly iohexol technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve the problems of high energy consumption and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] A kind of iohexol [5-[N-(2,3-dihydroxypropyl) acetamido]-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo -1,3-phthalamide] an energy-saving and environment-friendly continuous preparation method, the steps are as follows:
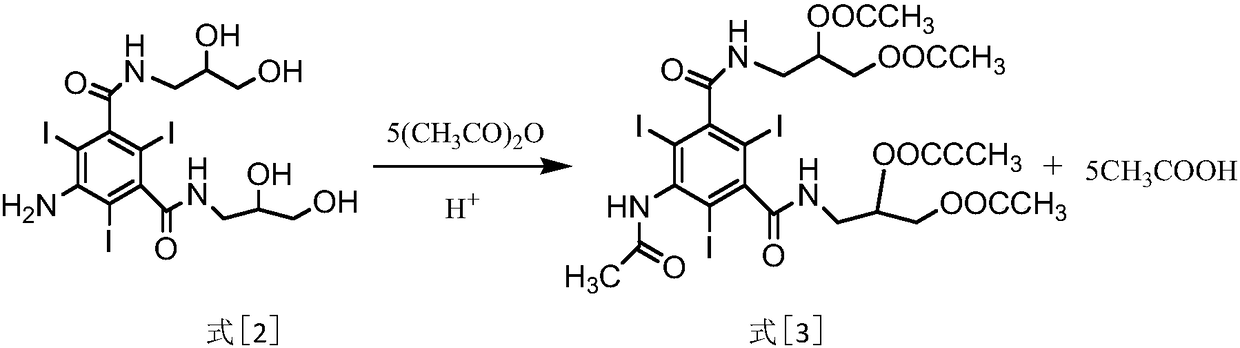
[0016] ⑴Acylation reaction: Formula [2] compound 5-amino-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)-1,3-phthalamide and ester The anhydride is reacted under the environment of hydrochloric acid to obtain the compound of formula [3] 5-acetylamino-2,4,6-triiodo-N,N'-bis(2,3-diacetoxypropyl)-1,3 - phthalamide;
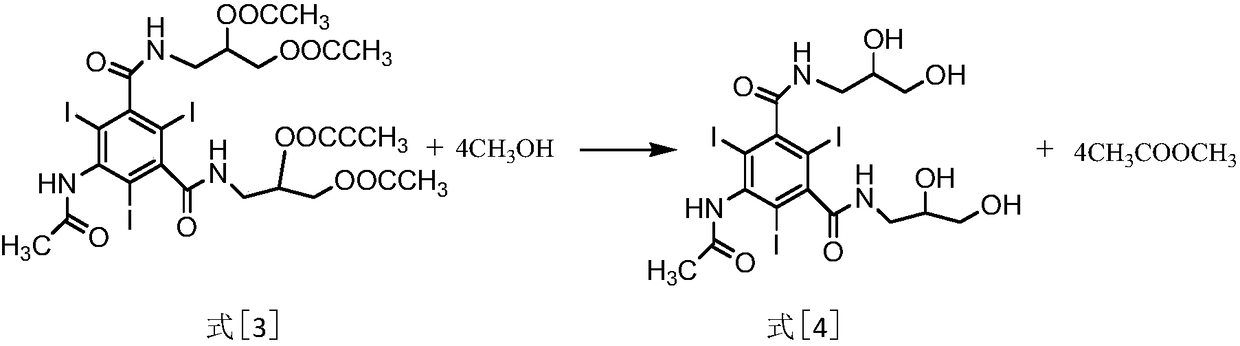
[0017] (2) transesterification reaction: the compound of formula [3] reacts with liquid alcohol under appropriate conditions to obtain the compound of formula [4] 5-acetylamino-2,4,6-triiodo-N,N'-bis(2,3 -dihydroxypropyl)-1,3-benzenedicarboxamide.
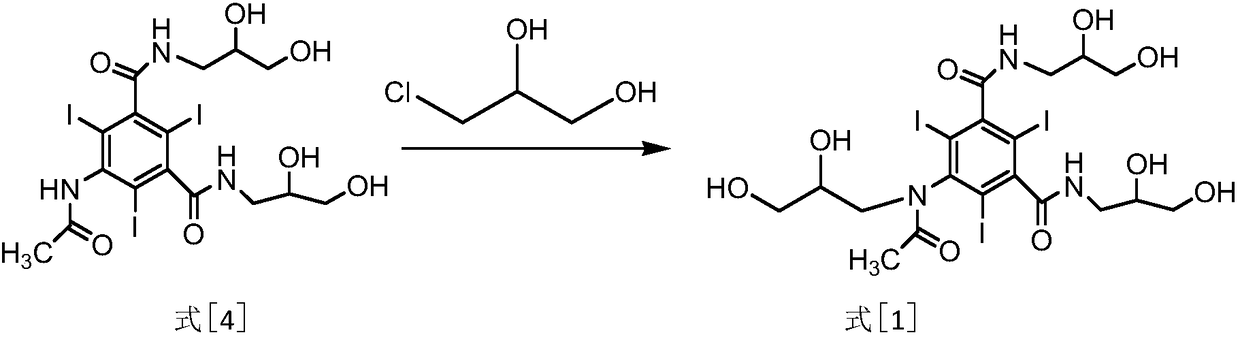
[0018] (3) Alkylation reaction: the compound of formula [4] reacts with 3-chloro-1,2-propanediol under the conditions of a mixture of methanol and sodium methylate to obtain the compound of formula [...
Embodiment 1
[0030] A continuous preparation method for energy saving and environmental protection of iohexol, the specific steps are as follows:
[0031] ⑴Acylation reaction: Formula [2] compound 5-amino-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)-1,3-phthalamide and ester For anhydride reaction, raise the temperature to 65°C-70°C and keep it warm for 2-6 hours. TLC monitors the completion of the reaction of the raw materials. Concentrate under reduced pressure and dry to obtain the sticky substance formula [3] compound 5-acetylamino-2,4,6-triiodo- N,N'-bis(2,3-diacetoxypropyl)-1,3-benzenedicarboxamide;
[0032] (2) Transesterification reaction: In the formula [3] compound 5-acetylamino-2,4,6-triiodo-N,N'-bis(2,3-diacetoxypropyl)-1,3-benzenedi Add methanol to formamide, use concentrated hydrochloric acid as a catalyst, and rectify for 3 hours. Light components such as methyl acetate are extracted from the gas phase, and the by-product methyl acetate is obtained by separation and purifica...
Embodiment 2
[0036] A continuous preparation method for energy saving and environmental protection of iohexol, the specific steps are as follows:
[0037] 2. (1) Starting with the compound 5-amino-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide of formula [2] As a raw material, take 30g of the compound and add it to a 250ml reaction flask, add 120ml of acetic anhydride and dropwise add 0.1g of concentrated hydrochloric acid. The temperature is raised to 65°C-70°C and the temperature is kept for 26h. The conversion rate of the raw material is not less than 97% as monitored by TLC. Concentrate under reduced pressure to remove part of acetic anhydride and acetic acid to obtain a mixture of the compound of formula [3], acetic anhydride and acetic acid. No need for purification, directly proceed to the next step reaction;
[0038] (2) Lower the temperature of the sticky substance formula [3] mixture obtained in the previous step to 70°C, slowly add anhydrous methanol under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com