Induced proliferation method of immune cell
A technology of immune cells and cells, applied in the field of induction and expansion of immune cells, can solve the problems of limited application of cell immunotherapy, poor uniformity of immune cells, high probability of tumorigenesis, etc., and achieve the improvement of induction differentiation efficiency and T cell expansion Efficiency improvement and uniformity improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
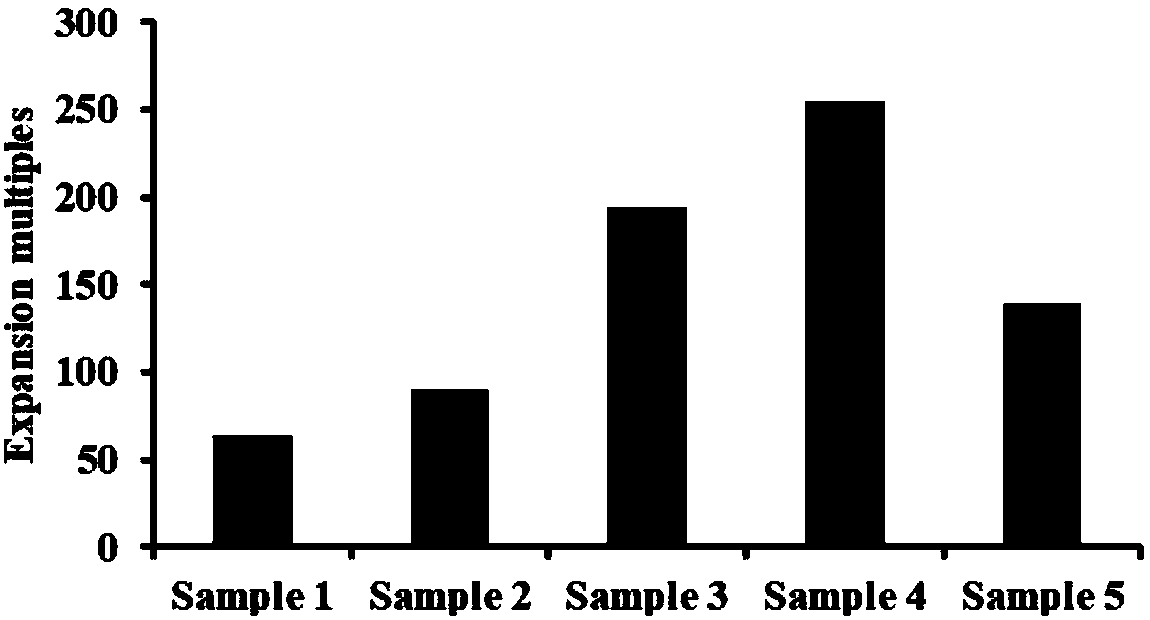
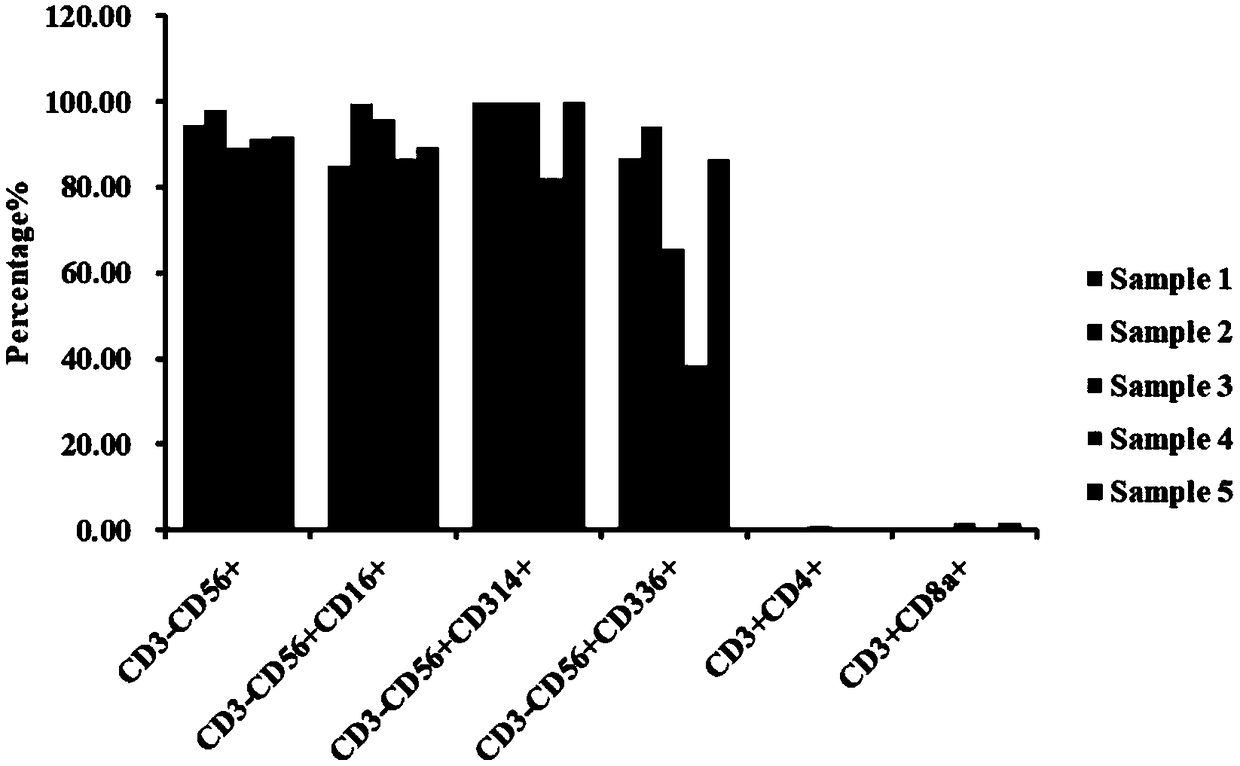
[0027] In this example, the inventors will describe in detail the method for inducing and expanding immune cells, including inducing and expanding isolated mononuclear cells into immune cells, and detecting the activity of immune cells.
[0028] 1. Experimental method
[0029] 1. Preparation of Anti-human CD16 Coated ZellWerk Bioreactors
[0030] 1.1 Add 15 mL of 2.5 μg / mL anti-human CD16 monoclonal antibody dissolved in medical normal saline to the bioreactor, shake the culture bottle gently to make the antibody cover the culture surface, and overnight at 4°C in the dark.
[0031] 1.2 Recover the antibody coating solution before use, wash the bioreactor once with 15mL of normal saline, and then use 15mL of T cell expansion medium (OpTmizer TM CTS TM T-cell expansion SFM) and washed once.
[0032] 2. Collect peripheral blood, separate peripheral blood plasma and mononuclear cells
[0033] 2.1 Collect about 100ml of human peripheral blood with a sterile blood collection bag...
Embodiment 2
[0076] In this example, the inventors will describe in detail the method for inducing and expanding immune cells, including inducing and expanding isolated mononuclear cells into immune cells, and detecting the activity of immune cells.
[0077] 1. Experimental method
[0078] 1. Preparation of Anti-human CD16 Coated ZellWerk Bioreactors
[0079] 1.1 Add 15 mL of 2.5 μg / mL anti-human CD16 monoclonal antibody dissolved in medical normal saline to the bioreactor, shake the culture bottle gently to make the antibody cover the culture surface, and overnight at 4°C in the dark.
[0080] 1.2 Recover the antibody coating solution before use, wash the bioreactor once with 15mL of normal saline, and then use 15mL of T cell expansion medium (OpTmizer TM CTS TM T-cell expansion SFM) and washed once.
[0081] 2. Collect peripheral blood, separate peripheral blood plasma and mononuclear cells
[0082] 2.1 Collect about 100ml of human peripheral blood with a sterile blood collection b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com