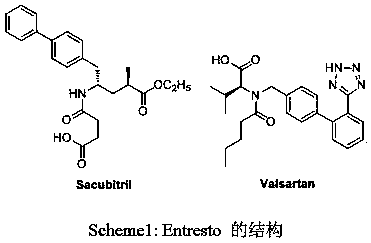
Synthesis of enkephalinase inhibitor
A technology of enkephalinase and inhibitors, which can be used in the preparation of carboxylic acid amide, cyanide reaction, organic compound, etc., and can solve the problems of high price and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
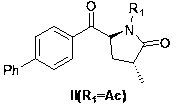
[0026] Embodiment 1: the synthesis of compound V (R1=Ac)
[0027] The synthesis of compound V (R1=Ac) refers to the literature (Journal of Organic Chemistry, 1986, vol. 51, p. 3494-3498) method. Add 104g (0.80mol, 1eq) L-pyroglutamic acid and 300g (2.88wt.) methanol to the three-necked flask, and add 138g (2.2eq) acetyl chloride dropwise. After the dropwise addition, the temperature was raised to 55-65°C until the reaction of the raw materials was complete. After the solvent was distilled off under reduced pressure to obtain a white solid, add 300g (2.88wt) toluene, dropwise add 178g (2.2eq) triethylamine, then add dropwise 69g (1.1eq) acetyl chloride, after the reaction is completed, add 160g water, stir After layering, the organic phase was washed successively with dilute hydrochloric acid, sodium bicarbonate solution, and water, and after concentration, 122 g of white solid was obtained.
[0028]
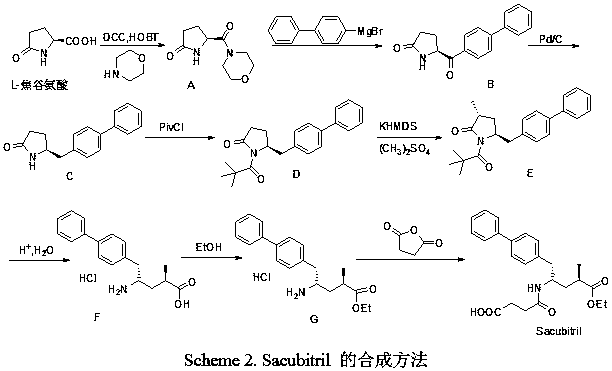
Embodiment 2
[0029] Embodiment 2: compound VI (R 1 =Ac) synthesis
[0030] Compound VI(R 1 =Ac) synthetic reference literature (Synthesis, 1997, 863-865) method. 50g (0.27mol, 1.0eq) compound V (R 1 =Ac, the product in Example 1), 70.6g (1.5eq) tert-butoxybis(dimethylamino)methane (Bredereck reagent), 200mL ethylene glycol dimethyl ether are added in the reaction flask, heated to React at 68-70°C for 10h. Cooling and crystallization, filtering, filter cake washing with a small amount of solvent, compound VI (R 1 =Ac), weight 46.3g.
[0031]
Embodiment 3
[0032] Embodiment 3: compound VII (R 1 =Ac) synthesis
[0033] Compound VII(R 1 =Ac) was obtained by referring to the literature (Synthesis, 1997, 863-865).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com