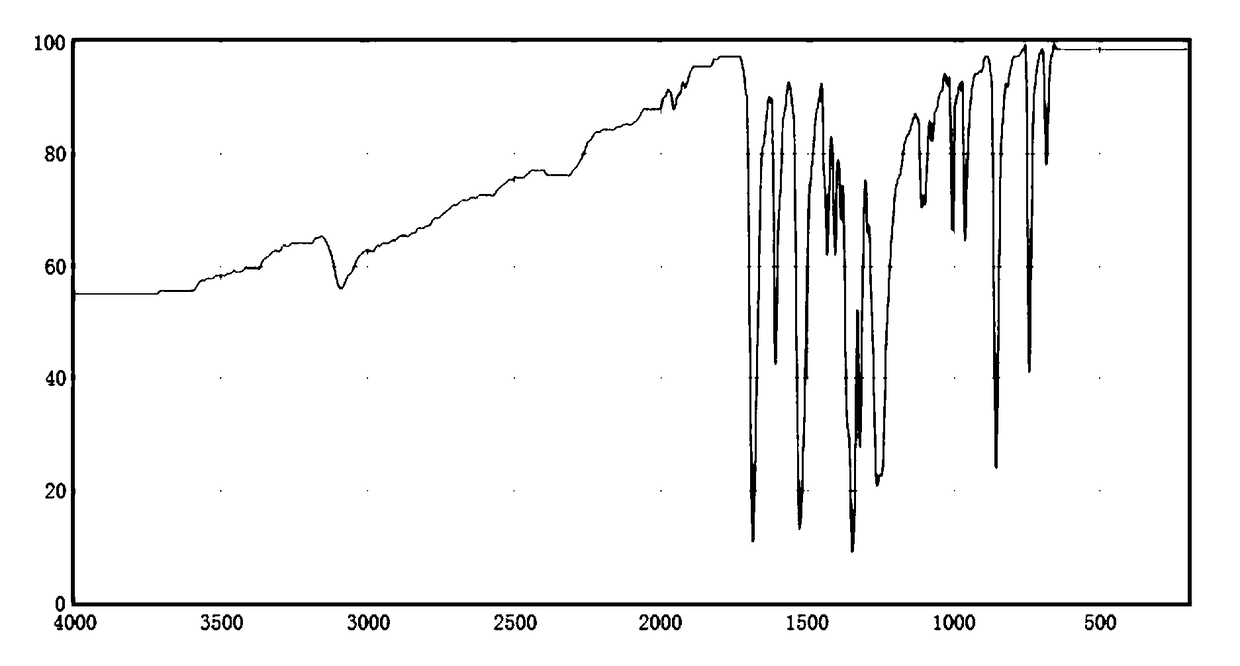
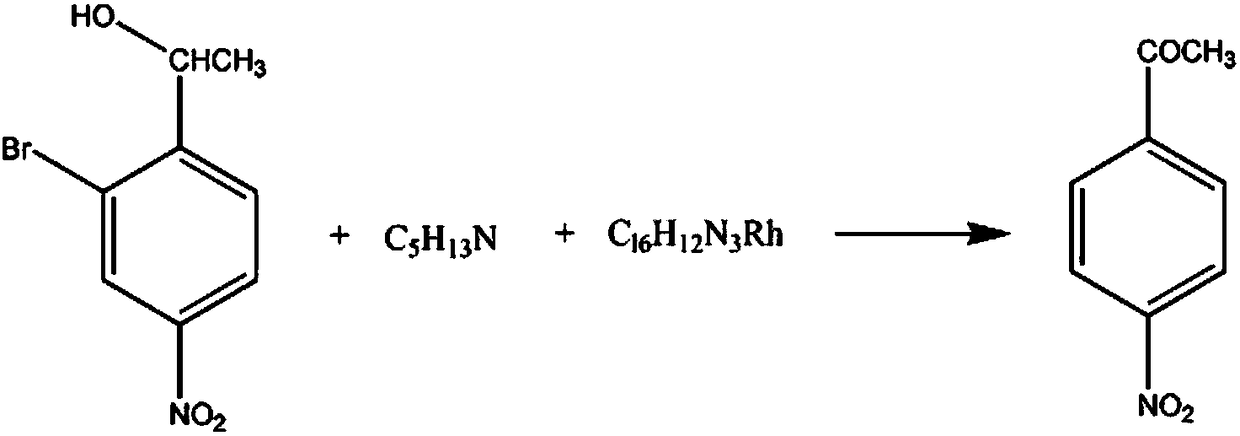Chloramphenicol drug intermediate p-nitroacetophenone synthesis method
A technology of p-nitroacetophenone and its synthesis method, which is applied in the field of organic synthesis, can solve problems such as complex process and low yield, and achieve the effects of increasing reaction yield, shortening reaction time, and reducing intermediate links in the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthetic method of chloramphenicol pharmaceutical intermediate p-nitroacetophenone, comprises the steps:
[0019] A. Add 3mol of 3-bromo-4-(1-hydroxyethyl)-nitrobenzene and 300ml of potassium bromide solution with a mass fraction of 10% into the reaction vessel, control the stirring speed at 160rpm, and add 4mol with a mass fraction of 55% 3-methylbutylamine solution, raise the temperature of the solution to 40°C, add 2mol of ammonium chlororhodate in 3 times, continue the reaction for 40min after the addition, and raise the temperature of the solution to 50°C;
[0020] B, adding mass fraction is 60% chlorinated isopentane solution to wash 3 times, mass fraction is 70% 4-chloro-1-butanol solution to wash 5 times, mass fraction is 30% potassium sulfate solution to wash 3 times , the solution is separated into layers, the oil layer is separated, the temperature is lowered to 10° C., recrystallized in a chlorodibutylene solution with a mass fraction of 90%, and dehydr...
Embodiment 2
[0022] The synthetic method of chloramphenicol pharmaceutical intermediate p-nitroacetophenone, comprises the steps:
[0023] A, 3-bromo-4-(1-hydroxyethyl)-nitrobenzene, 400ml mass fraction of 13% potassium bromide solution is added to the reaction vessel, the stirring speed is controlled at 170rpm, and 4.5mol mass fraction is added to be 60 % of 3-methylbutylamine solution, raise the solution temperature to 45°C, add 2.5mol ammonium chlororhodiumate in 5 times, continue to react for 50min after adding, raise the solution temperature to 53°C;
[0024] B, adding mass fraction is 62% chloroisopentane solution to wash 4 times, mass fraction is 73% 4-chloro-1-butanol solution to wash 6 times, mass fraction is 32% potassium sulfate solution to wash 5 times , the solution was separated into layers, the oil layer was separated, the temperature was lowered to 13° C., recrystallized in a chlorodibutylene solution with a mass fraction of 93%, and dehydrated with anhydrous calcium sulfat...
Embodiment 3
[0026] The synthetic method of chloramphenicol pharmaceutical intermediate p-nitroacetophenone, comprises the steps:
[0027] A. Add 3-mol of 3-bromo-4-(1-hydroxyethyl)-nitrobenzene and 500 ml of potassium bromide solution with a mass fraction of 16% into the reaction vessel, control the stirring speed at 190 rpm, and add 5 mol with a mass fraction of 63% 3-methylbutylamine solution, raise the temperature of the solution to 50°C, add ammonium chlororhodate in 5 times, continue the reaction for 60min after the addition, and raise the temperature of the solution to 57°C;
[0028] B, adding mass fraction is 65% chloroisopentane solution to wash 6 times, mass fraction is 77% 4-chloro-1-butanol solution to wash 7 times, mass fraction is 35% potassium sulfate solution to wash 6 times, The solution was separated into layers, the oil layer was separated, the temperature was lowered to 15° C., recrystallized in a chlorodibutylene solution with a mass fraction of 96%, and dehydrated wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com