Prescription and preparation method of multi-valent pneumococcal conjugate combined vaccine
A pneumococcal and combination vaccine technology, which is applied in the field of multivalent pneumococcal conjugate combination vaccines, can solve problems such as induction of unfavorable carrier antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
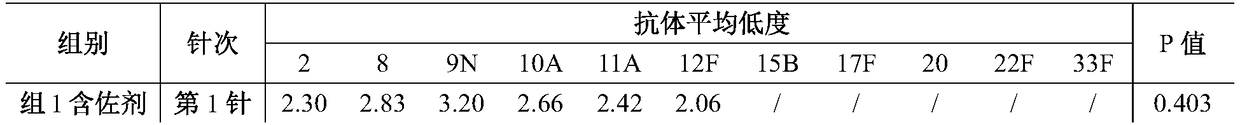
[0045] Example 1: Combination formulation of multivalent pneumococcal conjugates
[0046] Each 0.5ml dose contains a total of 22 μg of various types of pneumococcal polysaccharides (total polysaccharides), of which 2 μg each of 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F and 33F. Various types of polysaccharides are combined with diphtheria toxoid and adsorbed on aluminum phosphate adjuvant (aluminum content 0.125 mg), and the solvent is physiological sodium chloride solution.
Embodiment 2
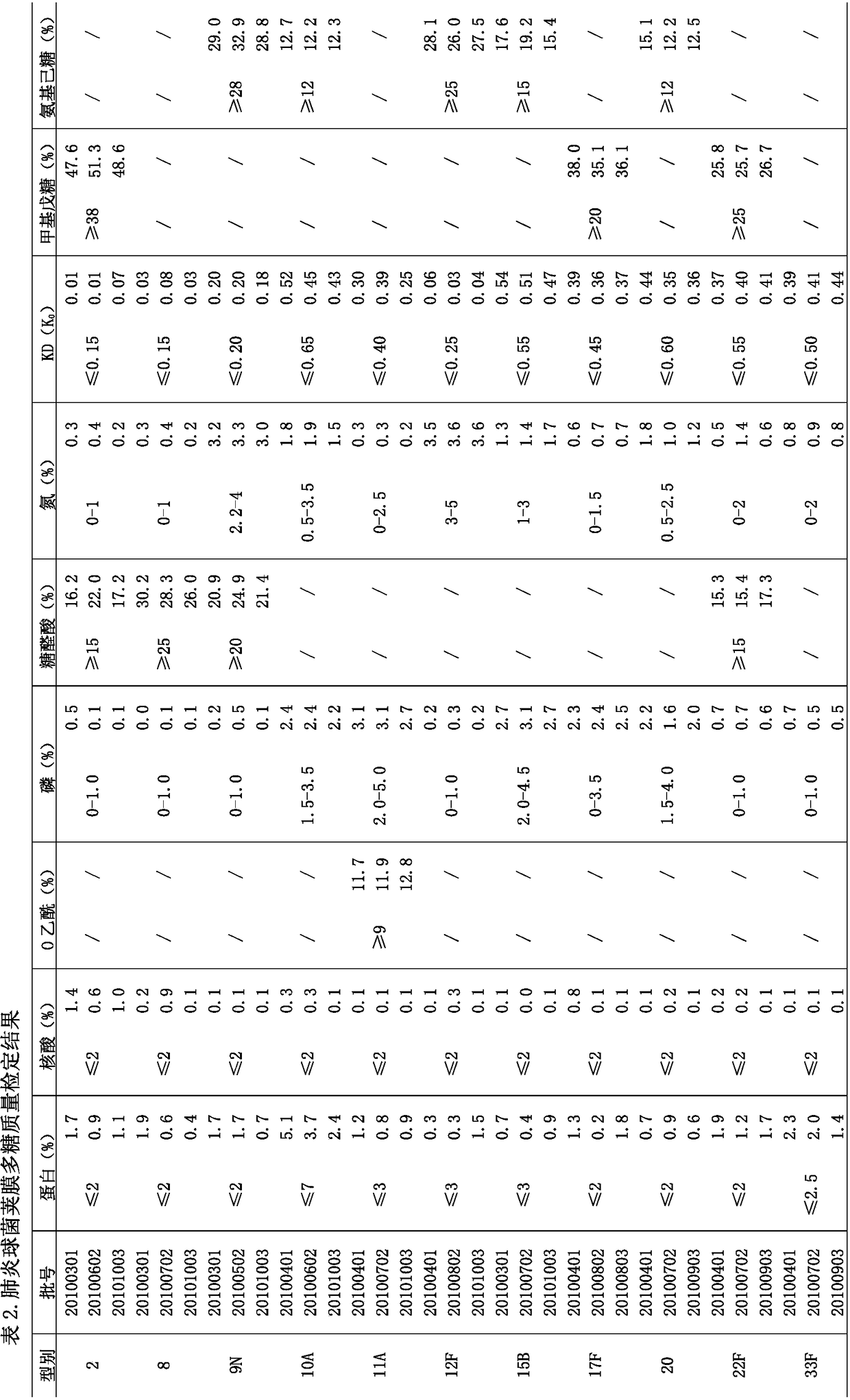
[0047] Embodiment 2: Purified pneumococcal polysaccharide assay result
[0048] The 11 types of pneumococcal capsular polysaccharides were purified separately, with 3 batches for each type, and the quality inspection results of each batch are shown in Table 2:
[0049]
Embodiment 3
[0050] Embodiment 3: Pneumococcal conjugate assay result
[0051] 11 types of pneumococcal conjugates prepared by the amine reduction method, various types of pneumococcal polysaccharides combined with diphtheria toxoid, 3 batches for each type, the test results of each batch of conjugates are shown in Table 3:
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com