Facile Synthesis of Porous Cyclodextrin Polymers for Efficient Removal of Antibiotics in Water
A technology of cyclodextrin polymer and synthesis method, applied in water pollutants, chemical instruments and methods, water/sewage treatment and other directions, can solve the problems of difficult reuse, poor adsorption capacity of antibiotics, etc. Low adsorption capacity, overcoming the effect of poor repeated adsorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A simple method for synthesizing a porous cyclodextrin polymer for efficiently removing antibiotics in water, specifically comprising the following steps:
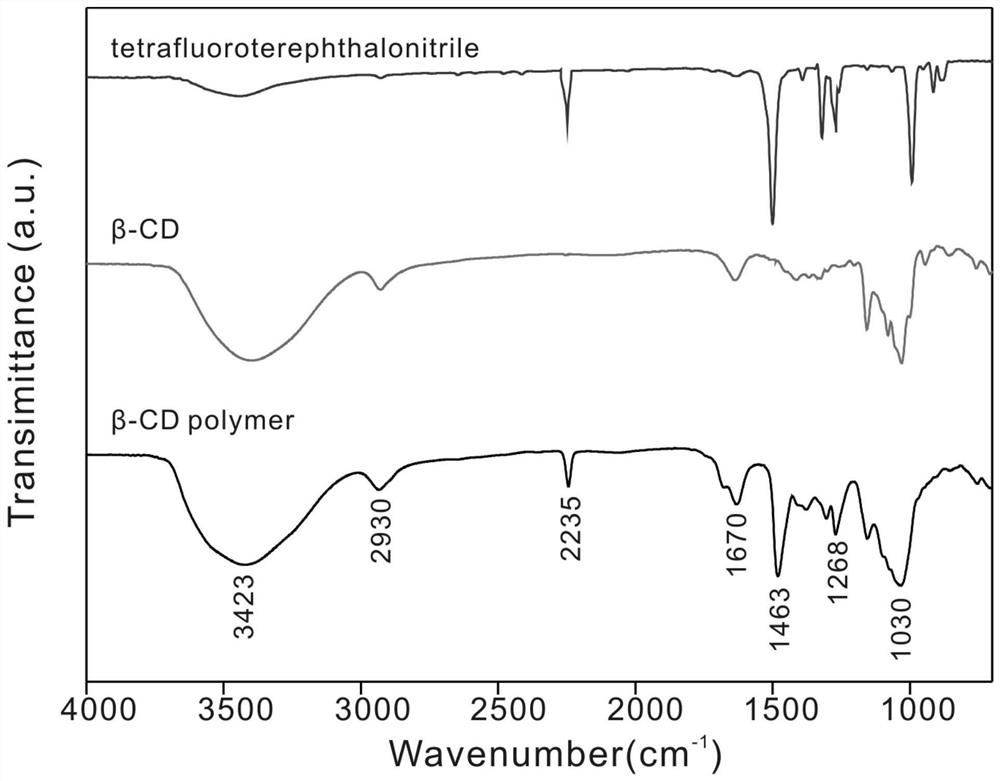
[0037] Step 1. Uniformly dissolve 8.0 g of soluble β-cyclodextrin powder, 4.0 g of tetrafluoroterephthalonitrile, and 12.8 g of potassium carbonate in 500 ml of dimethylformamide solvent to obtain a reaction precursor solution;
[0038] Step 2, reacting the obtained reaction precursor solution in an oil bath at 85°C for 2 days;
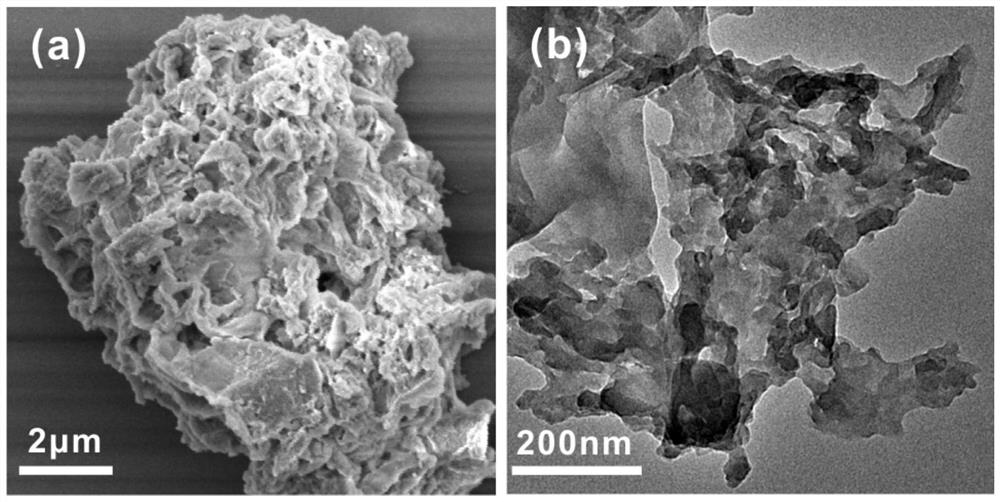
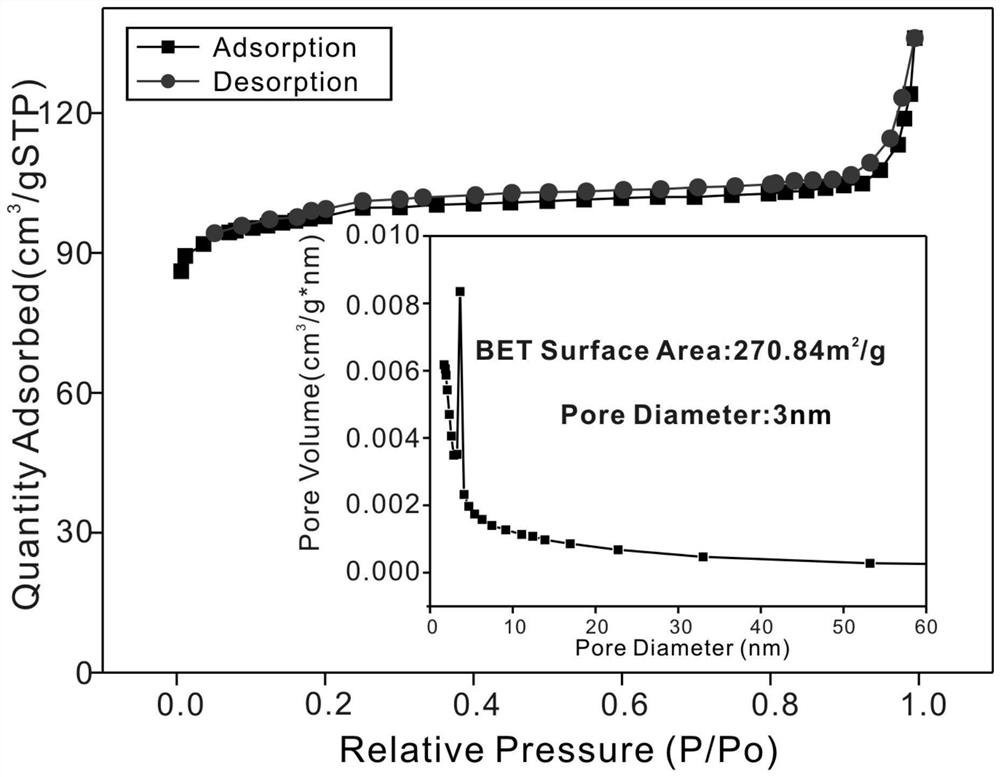
[0039] Step 3. After the polymerization reaction, cool naturally, separate and extract the porous β-cyclodextrin polymer through a simple suction filtration process, dry at 60°C, and grind it into powder after drying to obtain the desired porous cyclodextrin polymer thing. The surface of the porous cyclodextrin polymer is rich in active hydroxyl groups, and the specific surface area can reach 270.84m 2 / g, and the removal efficiency of tetracycline, doxycycline, levofloxacin, gatifloxacin...
Embodiment 2
[0047] A simple method for synthesizing a porous cyclodextrin polymer for efficiently removing antibiotics in water, specifically comprising the following steps:
[0048] Step 1. Uniformly dissolve soluble 5g of β-cyclodextrin, 3g of terephthalic acid, and 10g of potassium carbonate in 350mL of N,N-dimethylacetamide solvent to obtain a reaction precursor solution;
[0049] Step 2, reacting the obtained reaction precursor solution in an oil bath at 80°C for 1.5 days;
[0050] Step 3. After the polymerization reaction, cool naturally, separate and extract the porous β-cyclodextrin polymer through a simple suction filtration process, dry at 60°C, and grind it into powder after drying to obtain the desired porous cyclodextrin polymer thing.
[0051] The specific surface area of the porous cyclodextrin polymer can reach 234.6m 2 / g, the pore size is 2.2nm, and the removal efficiencies of tetracycline, doxycycline, levofloxacin, gatifloxacin and other four common antibiotics in ...
Embodiment 3
[0053] A simple method for synthesizing a porous cyclodextrin polymer for efficiently removing antibiotics in water, specifically comprising the following steps:
[0054] Step 1. Uniformly dissolve 10 g of γ-cyclodextrin, 4.5 g of trimesic acid, and 13 g of sodium carbonate in 500 mL of N,N-dimethylformamide solvent to obtain a reaction precursor solution;
[0055] Step 2, reacting the obtained reaction precursor solution in an oil bath at 88°C for 2.5 days;
[0056] Step 3. After the polymerization reaction, cool naturally, separate and extract the porous β-cyclodextrin polymer through a simple suction filtration process, dry at 60°C, and grind it into powder after drying to obtain the desired porous cyclodextrin polymer thing.
[0057] The specific surface area of the porous cyclodextrin polymer can reach 286.5m 2 / g, the pore size is 3.5nm, and the removal efficiencies of tetracycline, doxycycline, levofloxacin, gatifloxacin and other four common antibiotics in water ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com