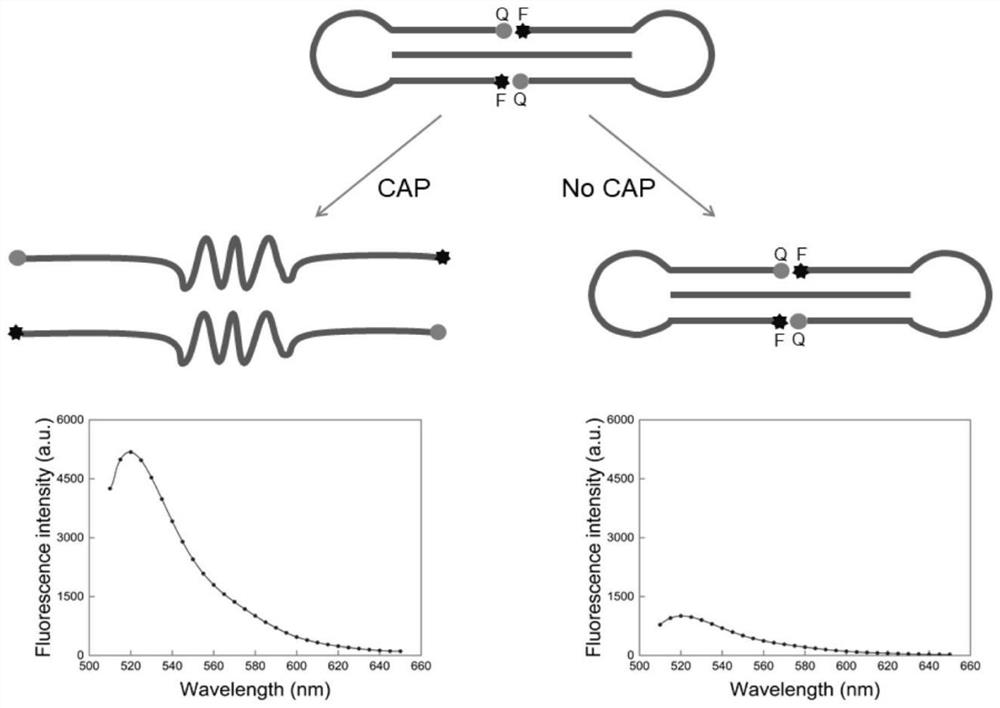
Double-head triple-helix nucleic acid probe and method for detecting chloramphenicol by using same
A nucleic acid probe and triple helix technology, applied in the field of antibiotic detection, can solve the problems of reducing the stability and reliability of the detection method and low sensitivity, and achieve the effects of wide sample range, high sensitivity and improved affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 double head triple helix nucleic acid probe
[0050] In this embodiment, the preparation of chloramphenicol-dependent double-ended triple-helical nucleic acid probe solution, the steps are as follows:
[0051] Add 7 μL of 10 μM circular probe stock solution and 3.5 μL of 10 μM circular probe stock solution to 3.5 μL of buffer (330 mM Tris-HCl, 660 mM KCl, 100 mM MgCl 2 , pH 7.5), add 17.5 μl of ultrapure water, shake well, and stand at room temperature for 15 minutes to obtain a chloramphenicol-dependent double-ended triple-helix nucleic acid probe solution.
[0052] Repeat the above operations to prepare several chloramphenicol-dependent double-ended triple-helical nucleic acid probe solutions, each 31.5 μL.
Embodiment 2
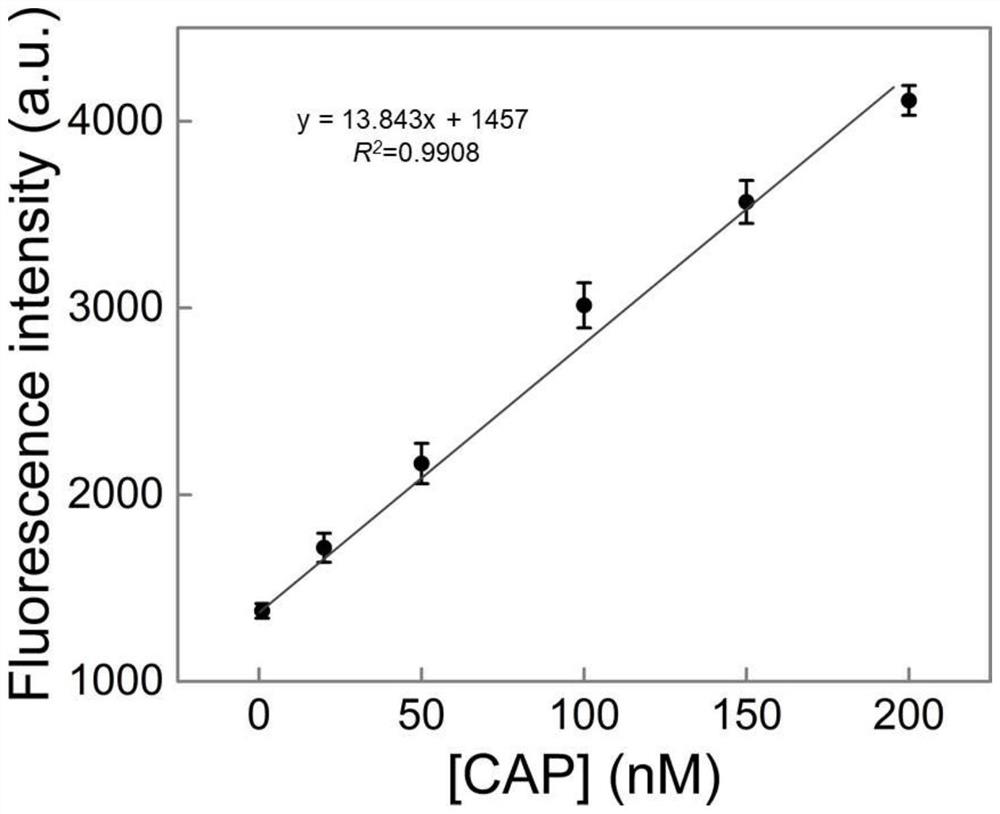
[0053] Embodiment 2 draws standard curve
[0054] In the present embodiment, the standard solution curve is drawn, and the steps are as follows:
[0055] (1) prepare chloramphenicol standard solution
[0056] Prepare chloramphenicol standard solutions with chloramphenicol concentrations of 0, 0.1, 0.5, 1, 20, 50, 100, 150, 200, 250, 300, and 500 nM, respectively.
[0057] (2) Draw a standard curve
[0058]① Take a portion (31.5 μL) of the chloramphenicol-dependent double-headed triple-helix nucleic acid probe solution prepared in Example 1, add 3.5 μL chloramphenicol standard solution with a chloramphenicol concentration of 0 nM to it, and place it at room temperature (25° C. ) placed for 27min to carry out the recognition reaction, then measure the fluorescence intensity of the reaction mixture, and record the peak value of the fluorescence intensity corresponding to the chloramphenicol standard solution;
[0059] ② Use chloramphenicol standard solutions with chloramphenic...
Embodiment 3
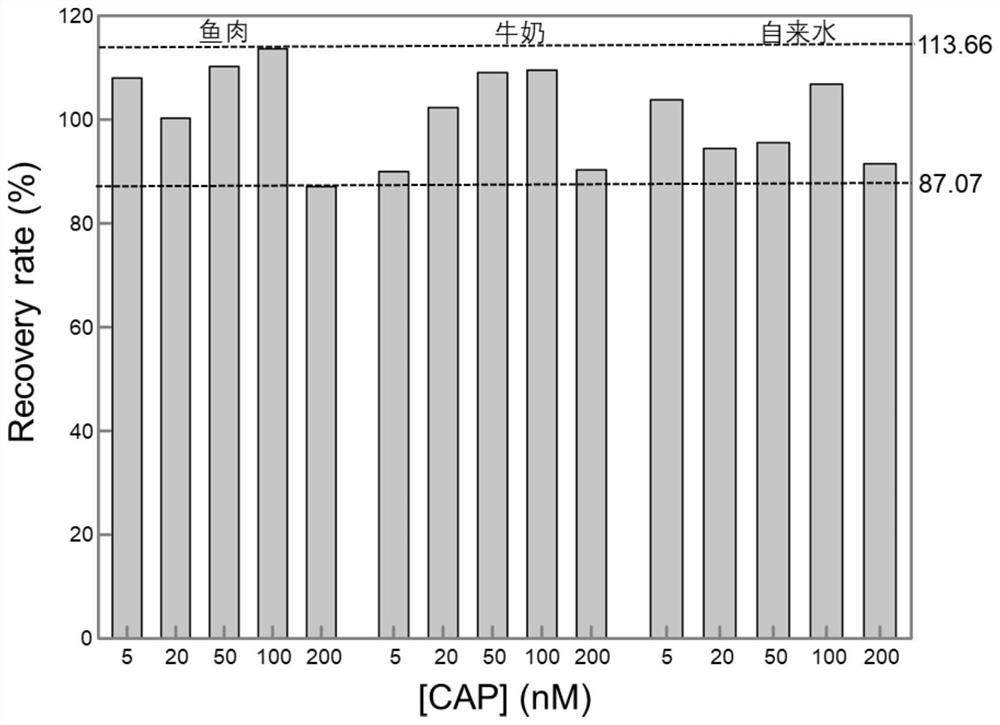
[0061] Embodiment 3 detects chloramphenicol in tap water
[0062] In this embodiment, chloramphenicol is added to tap water and the concentration of chloramphenicol is detected, the steps are as follows:
[0063] (1) Take tap water to prepare chloramphenicol sample solutions with chloramphenicol concentrations of 0, 5, 20, 50, 100, and 200 nM, respectively, and record them as 1# to 6# sample solutions in sequence.
[0064] (2) Chloramphenicol sample solution detection
[0065] ① Take a portion (31.5 μL) of the chloramphenicol-dependent double-headed triple-helix nucleic acid probe solution prepared in Example 1, add 3.5 μL of 1# sample solution to it, and place it at room temperature (25° C.) for 27 minutes to carry out the recognition reaction, and then Measure the fluorescence intensity of the reaction mixture, and record the peak fluorescence intensity corresponding to the chloramphenicol standard solution;
[0066] ②Use 2#~6# sample solution in turn to replace 1# sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com