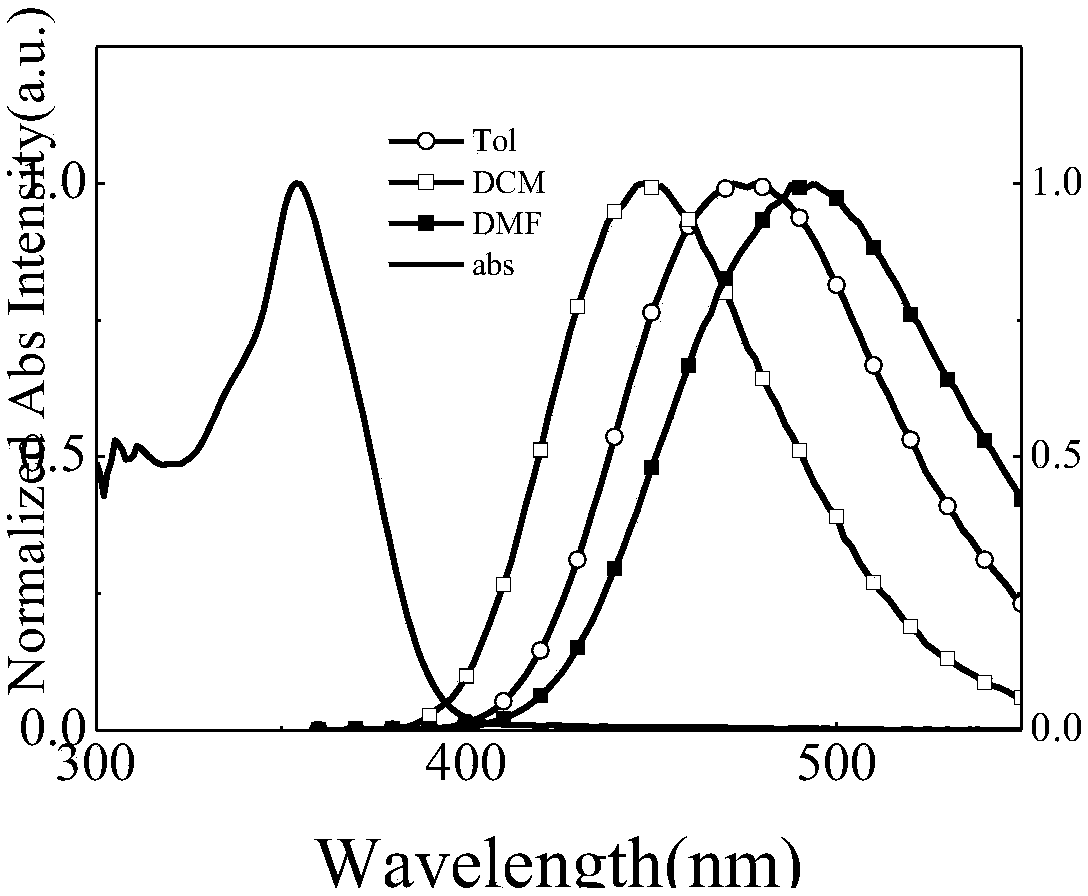
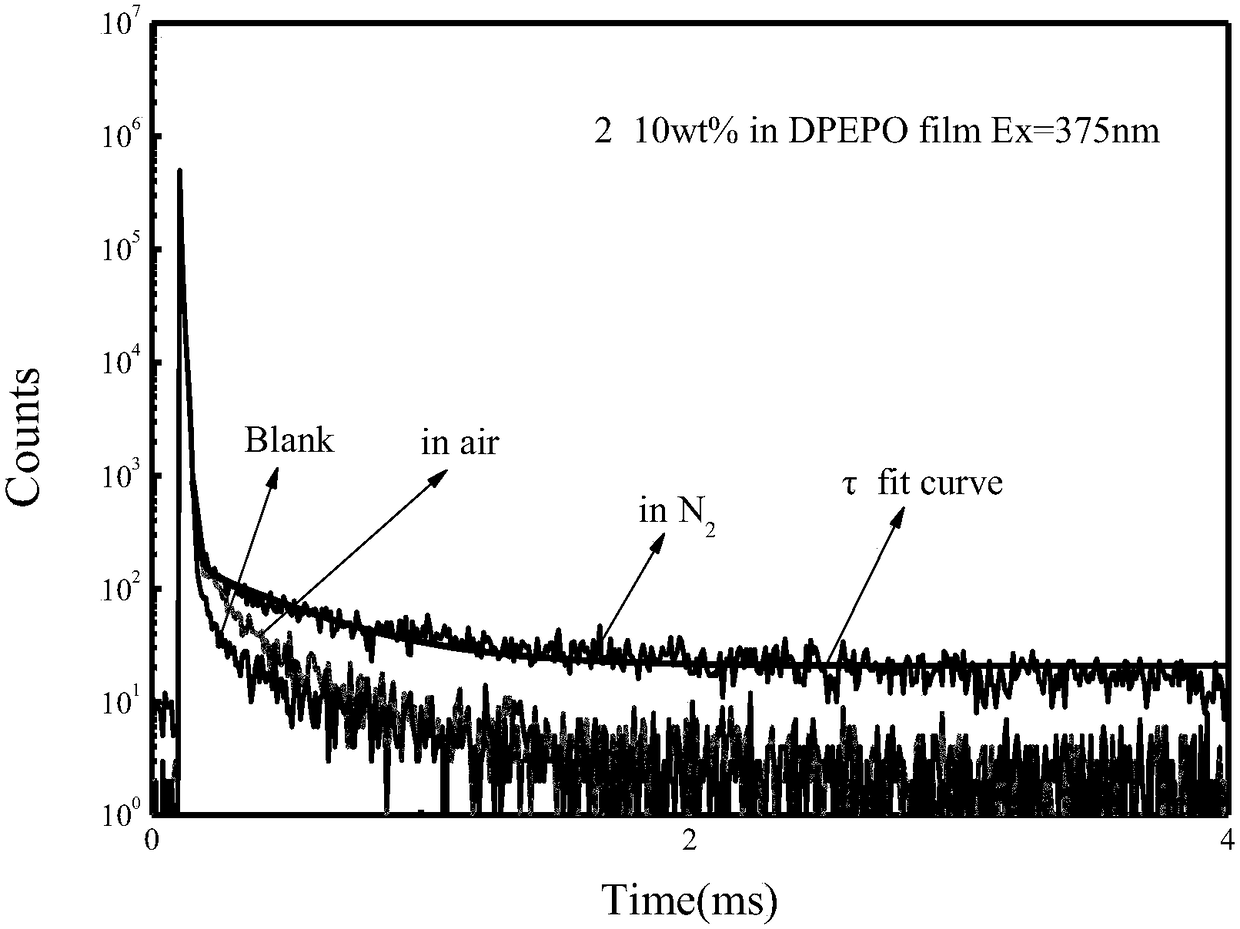
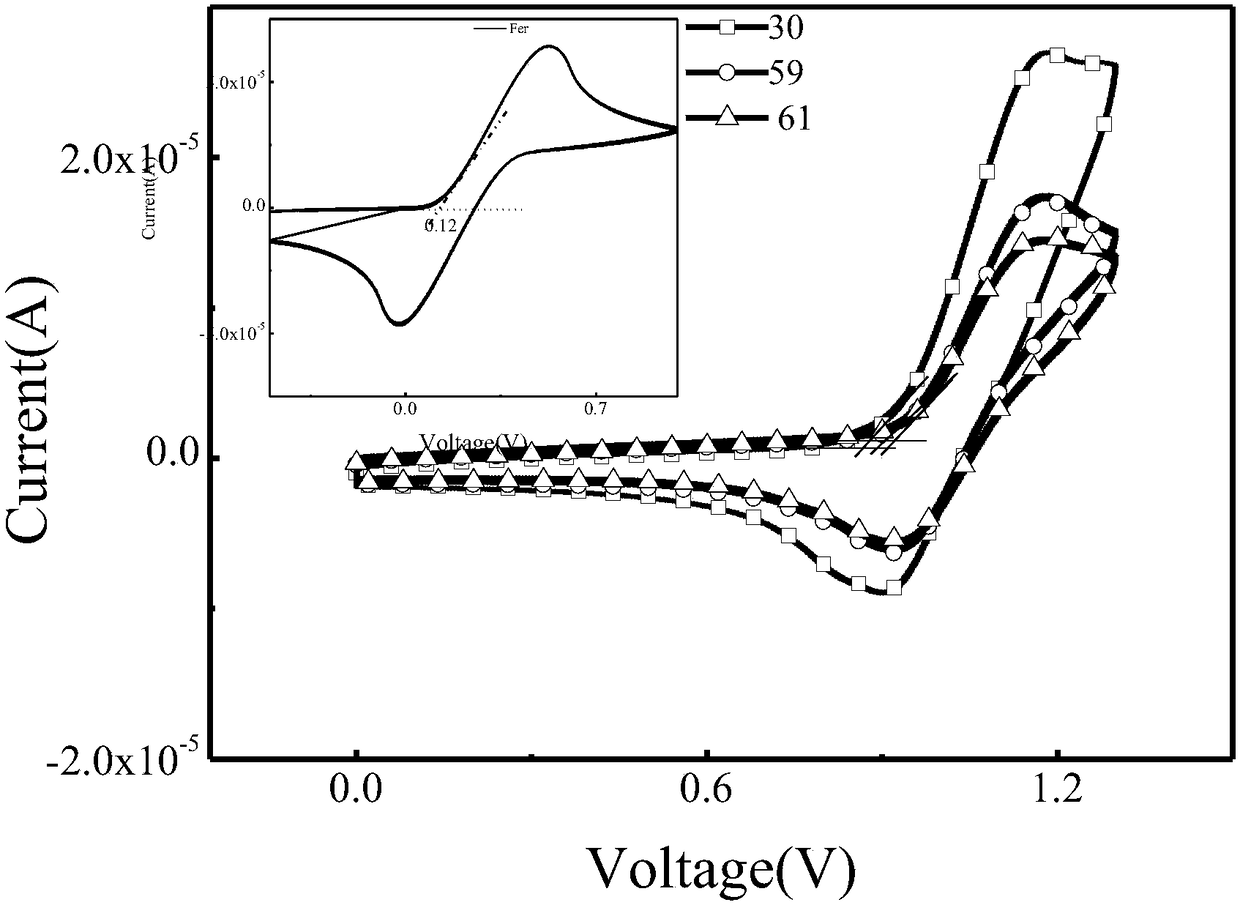
Miazines deviate and application thereof
A technology of pyrimidine derivatives, applied in the field of pyrimidine derivatives and their applications, can solve problems such as low efficiency and poor stability of blue light materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of pyrimidine derivatives in the present invention comprises the following steps:
[0035] One, the synthesis of various intermediates needed for pyrimidine derivatives in the present invention:
[0036] Intermediates (1), (2) can adopt the following synthetic route:
[0037] (1):
[0038] (2):
[0039] Taking the synthesis of intermediate (1) (5H-benzofuro[3,2-c]carbazole) as an example, the following steps are included:
[0040] ① Preparation of 4-boronic acid dibenzofuran (dibenzo[b,d]furan-4-ylboronic acid):
[0041] 4-Bromodibenzofuran (1eq), add dry tetrahydrofuran solution, cool down to -78°C, replace N 2 Three times, to ensure the oxygen-free environment of the system, after adding n-butyllithium (2.5M, 1.1eq) dropwise, keep it at -78°C for 1.5h, then add triisopropyl borate (ρ=0.815g / ml, 1.1 eq), rise to room temperature after the dropwise addition, drop dilute hydrochloric acid to quench the reaction after 8h, distill off THF un...
Embodiment 1
[0080] Taking the asymmetric pyrimidine derivatives shown in formula 2 as an example, the following synthetic route is adopted:
[0081]
[0082] (E)-1-(2-bromophenyl)-3-phenyl-prop-2-en-1-one ((E)-1-(2-bromophenyl)-3-phenylprop-2-en-1 -one) preparation:
[0083] Add a solution of 7g of NaOH and 50ml of water into a 250ml three-neck flask, add 25g of ethanol, stir vigorously, then dropwise add 18.5g of o-bromobenzaldehyde (2-bromobenzaldehyde, 100mmol), and add dropwise 12g of benzene after 30 minutes. Acetophenone (acetophenone, 100mmol), stir at 15-30°C for 2-3h, until light yellow solid appears, stirring is difficult, filter, wash with water to remove alkali until neutral, then wash with ice ethanol, distill the filter cake to remove ethanol under reduced pressure , to obtain 26 g of yellow sticky solid, yield 90%;
[0084] Preparation of (2-bromophenyl)-2,6-diphenylpyrimidine (4-(2-bromophenyl)-2,6-diphenylpyrimidine):
[0085] (E)-1-(2-bromophenyl)-3-phenyl-prop-2-e...
Embodiment 2
[0090] Taking the asymmetric pyrimidine derivatives shown in formula 5 as an example, the following synthetic route is adopted:
[0091]
[0092] 5-(4-(6-Chloro-2-phenylpyrimidin-4-yl)phenyl)-5H-benzofuro[3,2-c]carbazole (5-(4-(6-chloro- Synthesis of 2-phenylpyrimidin-4-yl)phenyl)-5H-benzofuro[3,2-c]carbazole):
[0093] 3.77g (4-(5H-benzofuro[3,2-c]carbazol-5-yl)phenyl)boronate ((4-(5H-benzofuro[3,2-c]carbazol-5 -yl)phenyl)boronic acid) (10mmol, 1eq) and 9g (22mmol, 4eq) of 4,6-dichloro-2-phenylpyrimidine, replace N 2 three times at N 2 Add 0.16g (0.1mmol, 1%eq) of palladium tetraphenylphosphine under the protection of 2M K 2 CO 3 Aqueous solution, ethanol, toluene (volume ratio = 1:2:1), 100ml in total, react in an oxygen-free environment for 12h, remove ethanol and toluene by distillation under reduced pressure, dissolve in dichloromethane, wash the organic phase with water, extract the aqueous phase, anhydrous Magnesium sulfate was dried, filtered, and the filtrate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com