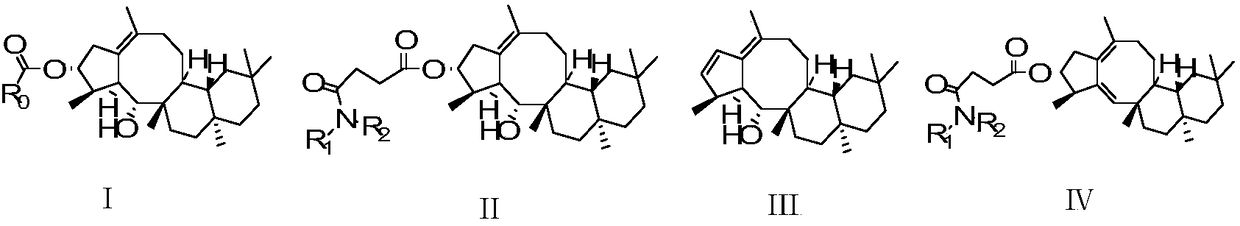
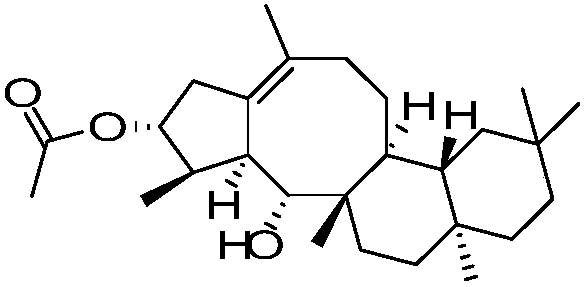
Sesterterpene AsperterpinolB derivatives sourced from marine microorganisms, synthetic method and anti-inflammatory application
A technology of sesquiterpene and derivatives, which is applied in the field of medicinal chemistry and can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] AsB-1:(2aS,6aS,6bS,12S,12aS,13R,13aS,Z)-2a,5,5,9,12,13a-hexamethyl-2,2a,3,4,5,6,6a, Synthesis of 6b,7,8,12,12a,13,13a-tetradecahydro-1H-cyclopenta[4,5]cycloocta[1,2-a]naphthalen-13-ol
[0030]
[0031] Experimental steps:
[0032]Weigh AsB (38.7mg, 0.103mmol, 1eq) in a 50ml round bottom flask, add 2ml of dichloromethane to dissolve, add boron trifluoride ether (mass fraction 46.5%-49.5%) dropwise into 1ml syringe (50ul, 0.135mmol , 1.3eq) in the solution, stirring at room temperature for half an hour, adding triethylamine to stop the reaction after half an hour, adjusting the pH to neutral, adding 15ml of saturated saline and dichloromethane (3×20ml) for extraction, and separating the organic phase, dried by adding anhydrous magnesium sulfate, and distilled under reduced pressure to obtain a crude product. Column chromatography in petroleum ether ethyl acetate system (V:V=1:10) gave 38.5 mg of a white solid with a yield of 95%.
[0033] White solid, yield 95%, m.p....
Embodiment 2
[0036] AsB-S 1 :(2aS,6aS,6bS,11R,12R,12aS,13R,13aS,Z)-13-hydroxy-2a,5,5,9,12,13a-hexamethyl-2,2a,3,4,5,6 ,6a,6b,7,8,10,11,12,12a,13,13a-hexadecahydro-1H-cyclopenta [4,5]cycloocta[1,2-a]naphthalen-11-yl acetate synthesis
[0037]
[0038] Experimental steps:
[0039] Weigh AsB (42.3mg, 0.11mmol, 1eq) in a 50ml two-necked round-bottom flask, add 2ml of anhydrous pyridine to dissolve, add anhydride (1.1mmol, 10eq), put it in an oil bath at 90°C to condense and reflux for reaction, and follow the reaction by TLC until The response is complete. Cool to room temperature and add 2M HCl solution to stop the reaction, adjust the pH of the solution to 2, stir for 15 minutes, add saturated brine 15ml and EA (3×15ml) to extract the organic phase, dry over anhydrous magnesium sulfate, and distill under reduced pressure to obtain the crude product, petroleum A pure white solid was obtained by column chromatography of ether ethyl acetate system (V:V=1:2).
[0040] White solid, yield 6...
Embodiment 3
[0043] AsB-S 2 :(2aS,6aS,6bS,11R,12R,12aS,13R,13aS,Z)-13-hydroxy-2a,5,5,9,12,13a-h examethyl-2,2a,3,4,5, Synthesis of 6,6a,6b,7,8,10,11,12,12a,13,13a-hexadecahydro-1H-cyclopenta[4,5]cycloocta[1,2-a]naphthalen-11-yl butyrate
[0044]
[0045] Experimental procedure: with embodiment 2
[0046] White solid, yield 72.04%, m.p.51.7-54.7℃. 1 H NMR (500MHz, CDCl 3 )δ4.75 (d, J = 6.5Hz, 1H), 4.57 (d, J = 10.7Hz, 1H), 3.01 (d, J = 1.8Hz, 1H), 2.78 (dd, J = 17.8, 6.5Hz, 1H), 2.62(t, J=14.8Hz, 1H), 2.22(ddd, J=18.3, 14.3, 7.0Hz, 4H), 1.73–1.65(m, 3H), 1.64(d, J=0.5Hz, 1H ),1.63(s,3H),1.62–1.49(m,2H),1.43–1.36(m,2H),1.35–1.24(m,6H),1.19–1.09(m,3H),1.08(s,3H ),0.97–0.92(m, 3H),0.91(s,3H),0.88(d,J=6.8Hz,6H),0.77(s,3H).
[0047] HRMS(ESI)for[M+Na] + :calcd for C 29 h 48 o 3 Na: 467.34957. Found: 467.3492.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com