Preparation method of florfenicol intermediate
A technology of florfenicol and intermediates, which is applied in the field of preparation of florfenicol intermediates, can solve the problems of many by-products, low yield and high atomic economic cost, and achieves reduction of by-product generation and improved yield , the effect of reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
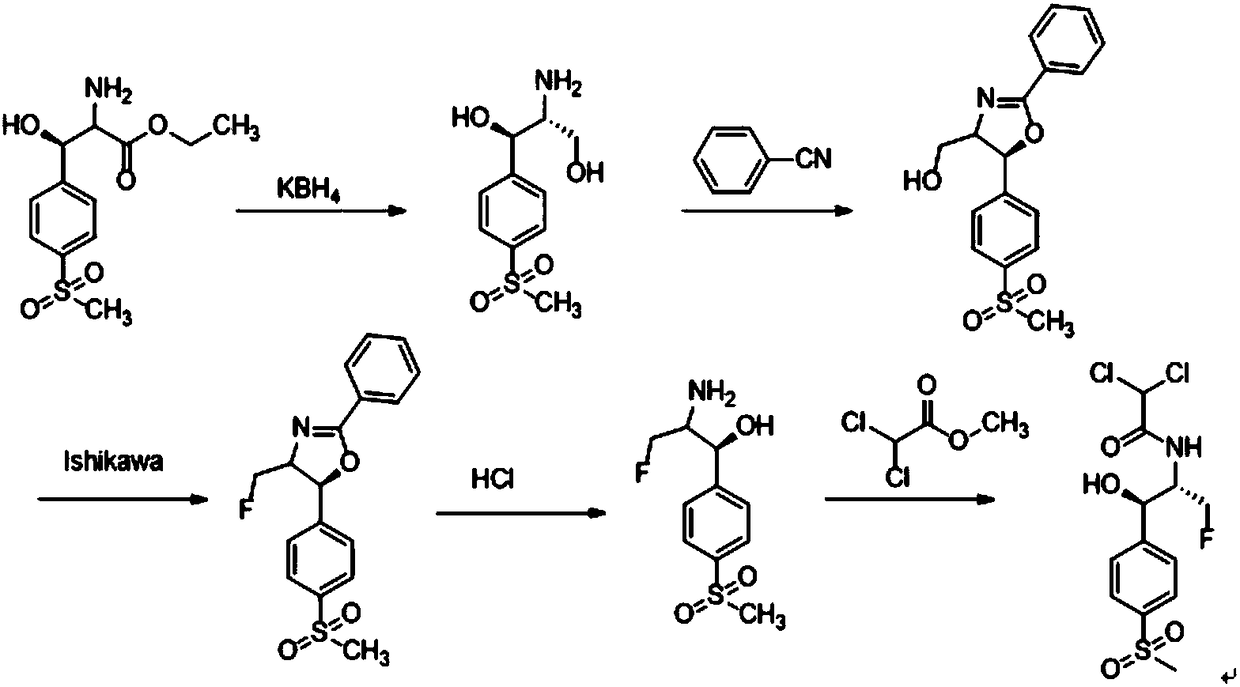
[0014] The invention provides a preparation method of florfenicol intermediate. The preparation method of the florfenicol intermediate includes the following steps:
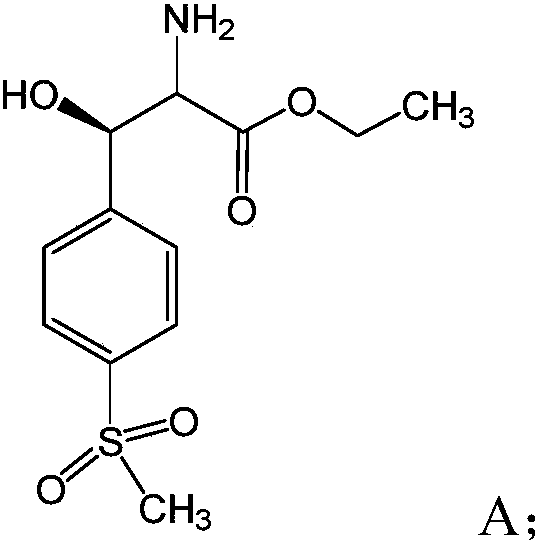
[0015] S01: Provide compound A with the following structural formula:
[0016]
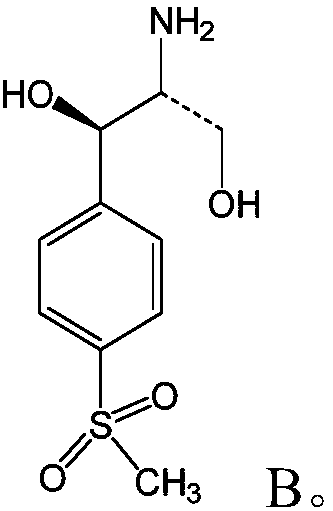
[0017] S02: The compound A is subjected to an oxidation-reduction reaction in a solvent containing an acridine salt photocatalyst and a reducing agent to generate compound B of the following structural formula:
[0018]
[0019] Specifically, the compound A in the step S01 is named D-(-)-threo-[p-(methylsulfonyl)phenyl]serine ethyl ester, which can be prepared according to conventional methods.
[0020] In the light reaction in the step S02, the compound A undergoes an oxidation-reduction reaction with the reducing agent under the action of light and the catalysis of the acridine salt photocatalyst to produce the compound B as a product. The chemical reaction formula of the redox reaction is as follows:
[0021]
[0022] Wherein, the acridin...
Embodiment 1
[0034] This embodiment provides a preparation method of florfenicol intermediate. The synthetic method of the florfenicol intermediate:
[0035] The compound A mentioned above is also D-(-)-threo-[p-(methylsulfonyl)phenyl]serine ethyl ester, the above-mentioned acridine salt visible light catalyst, diisopropylethyl The amine was added to anhydrous dichloroethane, and then the reaction environment was replaced with nitrogen three times, irradiated with a blue LED, and the reaction time was 20h. After the reaction, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 65%.
[0036] Wherein, the compound A, acridine salt, diisopropylethylamine and anhydrous dichloroethane are added in the following proportions: for every 2mL of anhydrous dichloroethane, add 0.2mmol, 1.0eq of the Compound A, 0.2mmol, 1.0eq of diisopropylethylamine, 0.02mmol, 0.1eq of acridine salt.
[0037] NMR and mass spectrometry o...
Embodiment 2
[0040] This embodiment provides a preparation method of florfenicol intermediate. The synthetic method of the florfenicol intermediate:
[0041] The compound A mentioned above is also D-(-)-threo-[p-(methylsulfonyl)phenyl]serine ethyl ester, the above-mentioned acridine salt visible light catalyst, diisopropylethyl The amine was added to anhydrous dichloroethane, and then the reaction environment was replaced with nitrogen three times, irradiated with a blue LED, and the reaction time was 20h. After the completion of the reaction, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 95%.
[0042] Wherein, the compound A, acridine salt, diisopropylethylamine and anhydrous dichloroethane are added in the following proportions: for every 2mL of anhydrous dichloroethane, add 0.2mmol, 1.0eq of the Compound A, 0.4mmol, 2.0eq of diisopropylethylamine, 0.02mmol, 0.1eq of acridine salt.
[0043] NMR and m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com