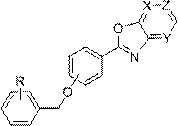
2-benzyloxyphenyloxazolopyridine compound and medicinal application thereof
A technology of benzyloxyphenyl oxazole and pyridine, applied in the field of medicinal chemistry, can solve the problems of short half-life, highly unstable structure, weak enzyme inhibitory activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
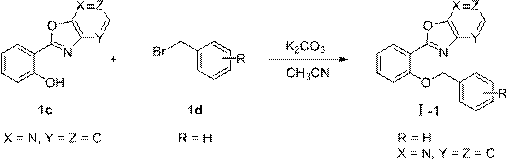
[0058] Example 1: Preparation of (2-(benzyloxy)phenyl)oxazolo[5,4-b]pyridine (Formula I-1)
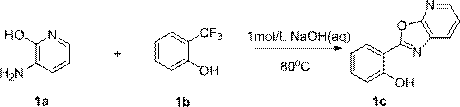
[0059] 1. Synthesis of (oxazolo[5,4-b]pyridin-2-yl)phenol (compound 1c)
[0060]
[0061] Dissolve 1.0 g (9.08 mmol, 1.05 eq) of 3-amino-2-hydroxypyridine in 34.6 mL of sodium hydroxide aqueous solution (1 mol / L, 34.60 mmol, 4.0 eq) at room temperature, stir well and add to it 1.4 g (8.65 mmol, 1.0 eq) 2-trifluoromethylphenol, 80 o C oil bath heating reaction for 2.0 h. TLC detects the disappearance of the raw materials, and stops heating. Cool to room temperature, extract with dichloromethane, combine the organic phases, wash with saturated sodium chloride solution, dry with anhydrous sodium sulfate, purify by PE-EA system column chromatography, and concentrate to obtain 1.40 g of compound 1c as a white solid with a yield of 76.3%;
[0062] After testing, the structure is correct and the testing results are as follows:
[0063] Compound 1c m.p. 153.7-154.5 o C; 1 H NMR (400 MHz, DMSO) δ 11....
Embodiment 2
[0069] Example 2: Synthesis of formula I-2, I-3, I-4, I-5, I-6, I-7, I-8, I-9, I-10, I-11, I-12, Compounds of I-13, I-17, I-18, I-19, I-20, I-21, I-22, I-23, I-24, I-25
[0070]
[0071] Formula I
R
Ⅰ-2
2-Cl
Ⅰ-3
3-Cl
Ⅰ-4
4-Cl
Ⅰ-5
4-CH 3
Ⅰ-6
2-F
Ⅰ-7
3-F
Ⅰ-8
4-F
Ⅰ-9
2-CH 3
Ⅰ-10
3-CH 3
Ⅰ-11
2-NO 2
Ⅰ-12
3-NO 2
Ⅰ-13
4-NO 2
Ⅰ-17
2-Et
Ⅰ-18
2-OCF 3
Ⅰ-19
3-OCF 3
Ⅰ-20
4-OCF 3
Ⅰ-21
2-CN
Ⅰ-22
3-CN
Ⅰ-23
4-CN
Ⅰ-24
3-F; 5-OCH3
Ⅰ-25
3,5-Difluoro
[0072] With reference to the conditions of the second step synthesis of compound formula I-1 in Example 1, from (oxazolo[5,4-b]pyridin-2-yl)phenol (compound 1c) and the corresponding commercially available substituted benzyl bromide (compound 2d ~ 13d and compound 17d ~ 25d) to obtain compound formula I-2 to I-13 and formula I-17 to I-25, specifically: (2-((2-chlorobenzyl)oxy)phenyl)oxa Azolo[5,4-b]pyridine (formula I-2); (2-((3-chlorobenzyl)oxy)phenyl)oxazolo[5,4-b]pyridine (formula I-3 ); (2-((4-...
Embodiment 3
[0095] Example 3: Synthesis of compounds of formula I-14, I-15, I-16, I-26, I-27
[0096] Synthesis of compounds 14d, 15d, 16d, 26d and 27d:
[0097]
[0098] Compound
R
14e
2-OCH 3
15e
3-OCH 3
16e
4-OCH 3
26e
2-CH 3 ; 3-F
27e
2-CH 3 ; 5-F
[0099] Dissolve 1.0 g (7.24 mmol, 1.0 eq) 3-fluoro-2-methylbenzaldehyde (compound 26e) in 15 ml absolute ethanol, and add 328.6 mg (8.69 mmol, 1.2 eq) NaBH 4 , React at room temperature for 3.0 h, add a small amount of water to quench the reaction, evaporate ethanol, add 15 ml of water, extract with EA (20 ml*2), wash twice with saturated sodium chloride solution, add anhydrous sodium sulfate and stir to dry. Concentrated to obtain 0.7 g of colorless and transparent oil (compound 26g), with a yield of 69.0%. According to the above reaction operation, compounds 14g, 15g, 16g and 27g were prepared from compounds 14e, 15e, 16e and 27e respectively;
[0100] Dissolve 0.7 g (4.99 mmol, 1.0 eq) of compound 26g in 15 ml CH 2 Cl 2 , Add 1.62 g (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com