Gastrin-releasing peptide precursor diluent, and application and kit thereof
A gastrin-releasing peptide and diluent technology, which is applied in biological testing, preparation of test samples, material inspection products, etc., can solve inaccurate detection, thermal stability, unsatisfactory stability of freeze-dried products, and poor thermal stability and other problems, to achieve the effect of good reproducibility, accurate detection results of gastrin releasing peptide precursor, and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
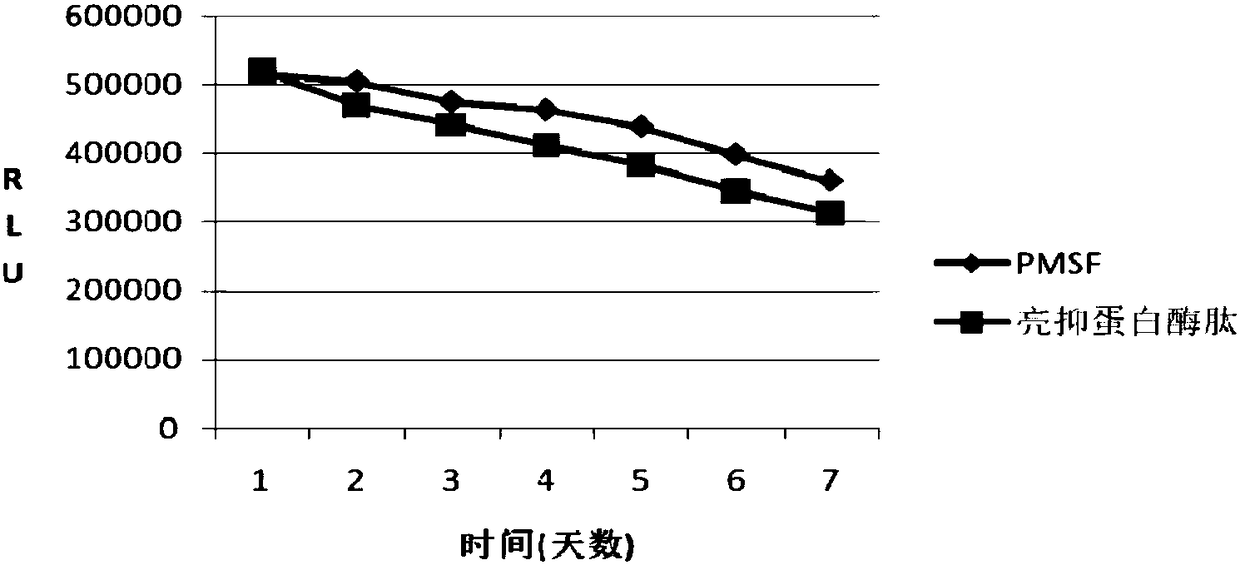
Examples
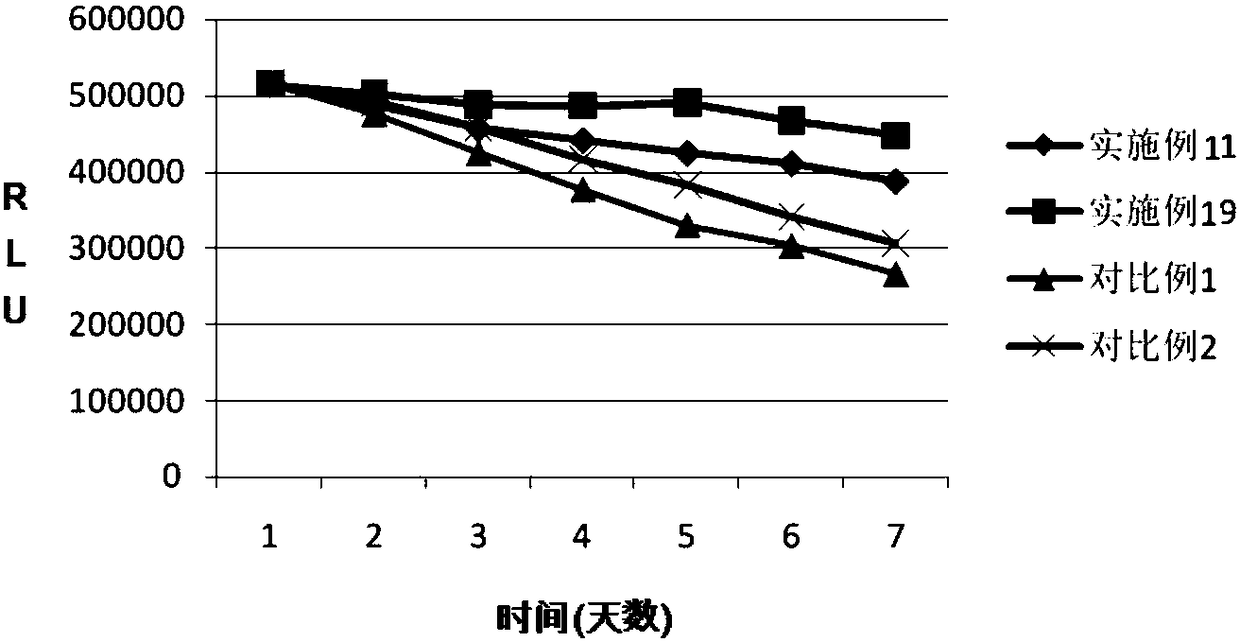
Embodiment 11
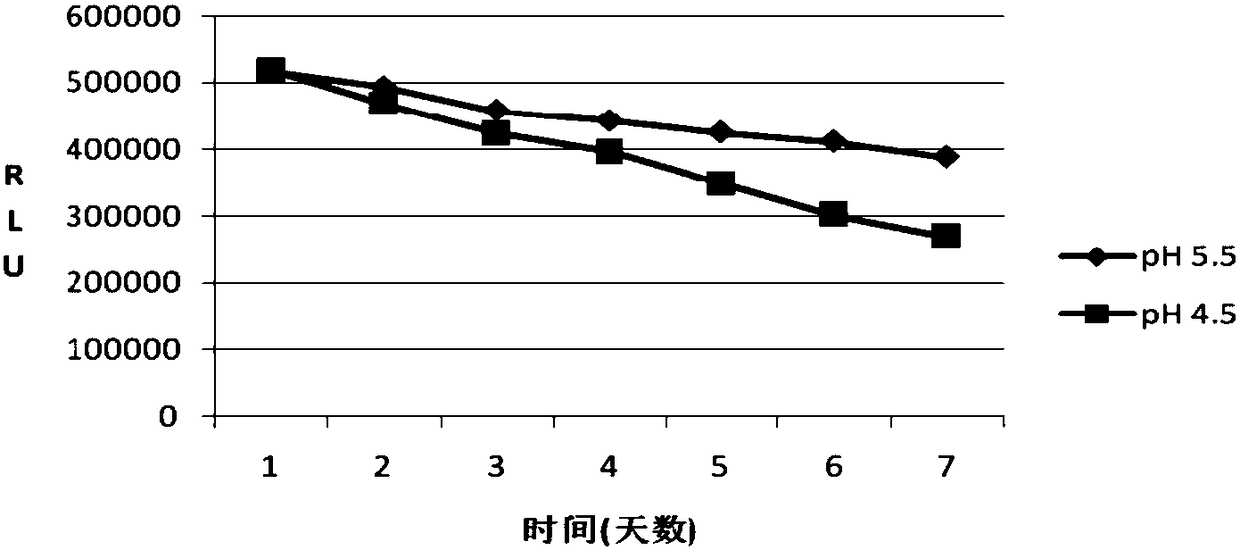
[0129] This embodiment provides a ProGRP dilution. The ProGRP dilution contains the components at the concentrations in Table 11 below, and the measured pH value is 5.5. It can be formulated as follows:
[0130] Taking 1000mL antigen diluent as an example, the dosage of the components is as follows:
[0131] Solution A: 0.1M citric acid diluent (21.01g)
[0132] Solution B: 0.1M sodium citrate dilution (29.41g)
[0133] Table 11
[0134] Preparation concentration
Embodiment 12
[0136] This embodiment provides a ProGRP dilution. The ProGRP dilution contains the components in the middle concentrations in Table 12 below, and the measured pH value is 4.5. It can be formulated as follows:
[0137] Taking 1000mL antigen diluent as an example, the dosage of the components is as follows:
[0138] Solution A: 0.1M citric acid diluent (21.01g)
[0139] Solution B: 0.1M sodium citrate dilution (29.41g)
[0140] Table 12
[0141] Preparation concentration
Embodiment 13
[0143] This embodiment provides a ProGRP dilution. The ProGRP dilution contains the components in the concentrations shown in Table 13 below, and the measured pH value is 5.5. It can be formulated as follows:
[0144] Taking 1000mL antigen diluent as an example, the dosage of the components is as follows:
[0145] Solution A: 0.1M citric acid diluent (21.01g)
[0146] Solution B: 0.1M sodium citrate dilution (29.41g)
[0147] Table 13
[0148] Preparation concentration
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com