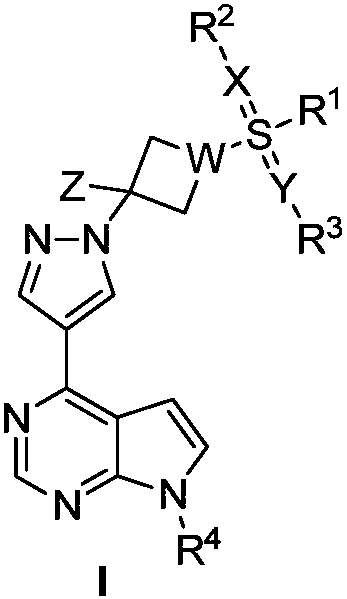
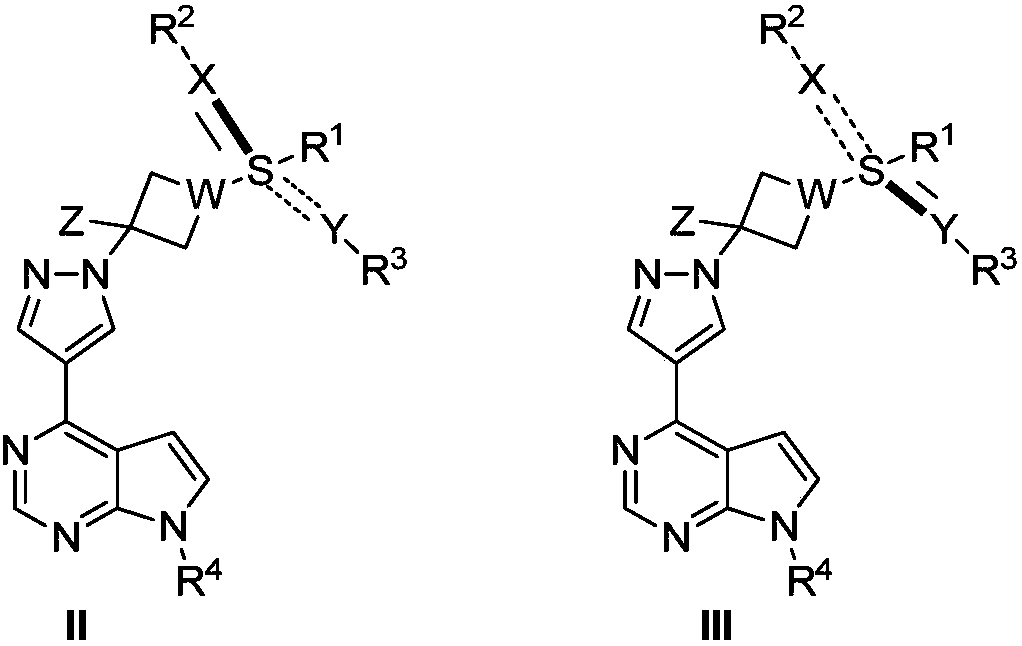
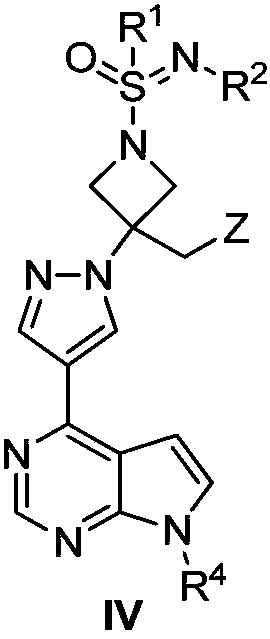
JAK (Janus kinase) inhibitor and preparation method and use thereof
A kind of technology of solvate and compound, applied in the field of JAK enzyme inhibitor and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1: the synthesis of compound 1a
[0152]
[0153] Under the protection of argon, 4-chloropyrrolopyridine (308g, 2.0mol, SM-1) was dissolved in 1.5L of anhydrous DMAc, cooled to 10°C in an ice-water bath, and NaH (104g, 2.6mol, 60%) was added in batches Maintain the temperature at 10-15°C, and stir at this temperature for 1 h after the dropwise addition is complete. A tetrahydrofuran solution of methyl pivalate (390 g, 2.6 mol dissolved in 1.5 L anhydrous THF, SM-2) was slowly added dropwise, keeping the temperature below 20° C., and the addition was completed after 1 h. The reaction solution was raised to room temperature for 2 h, and TLC showed that the reaction was complete. Water was added dropwise in an ice-water bath to quench the reaction, and extracted with methyl tert-butyl ether. Concentration of the organic phase afforded 1a (530 g) as a white solid.
Embodiment 2
[0154] Embodiment 2: the synthesis of compound 2a
[0155]
[0156] Compound 1a (309g, 1.16mol), 1-(1-ethoxyethyl)-4-pyrazoleboronic acid pinacol ester (339g, 1.28mol, SM-3), cesium fluoride (352g , 2.32mol), n-butanol (1.5L), water (1.5L). After evacuating argon three times, tetrakis(triphenylphosphine)palladium (10 g, 12 mmol) was added. After the argon was replaced again, the temperature was raised to reflux, and the reaction was carried out for 16 hours. HPLC showed that the reaction was complete. After cooling down, the liquid was separated, extracted with ethyl acetate, concentrated and directly proceeded to the next step (containing n-butanol).
Embodiment 3
[0157] Embodiment 3: the synthesis of compound 3a
[0158]
[0159] Compound 2a was dissolved in 500 mL of tetrahydrofuran, 4N hydrochloric acid solution (800 mL) was added dropwise, and reacted overnight at room temperature. HPLC showed that the reaction of the raw materials was complete, and the purity of the target compound was 73.1%. Slowly add 10% sodium hydroxide solution dropwise under ice-water bath until pH = 7-8, a lot of solids are formed. After filtering, the filter cake was washed with water and dried in an oven to obtain solid 3a (193 g), with a purity of 92% and a two-step yield of 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com