Application of ammonium chloride for separating uranium dioxide and lanthanide oxide
A technology of lanthanide elements and uranium dioxide, which is applied in the application field of ammonium chloride in the separation of uranium dioxide and lanthanide oxides, can solve the problems of high melting and boiling point, long chlorination time, and difficult separation, and achieves The effect of mild reaction conditions, low equipment requirements and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: UO 2 Dissolution Test in Chloride Molten Salt System
[0036] UO 2 For the dissolution process in the chloride molten salt system see figure 1 .
[0037](1) Mix 8.8g LiCl and 11.2g KCl evenly, place in an alumina crucible, place in a vacuum oven, dry at 150°C for more than 72 hours, and set aside.
[0038] (2) Add 0.14g UO 2 powder with 2g NH 4 The Cl powder was mixed evenly, and spread on top of the LiCl-KCl mixed salt at room temperature.
[0039] (3) Heat the high-temperature resistance furnace to 500°C, then place the corundum crucible containing the mixture in the resistance furnace, and keep a constant temperature of 500°C for reaction.
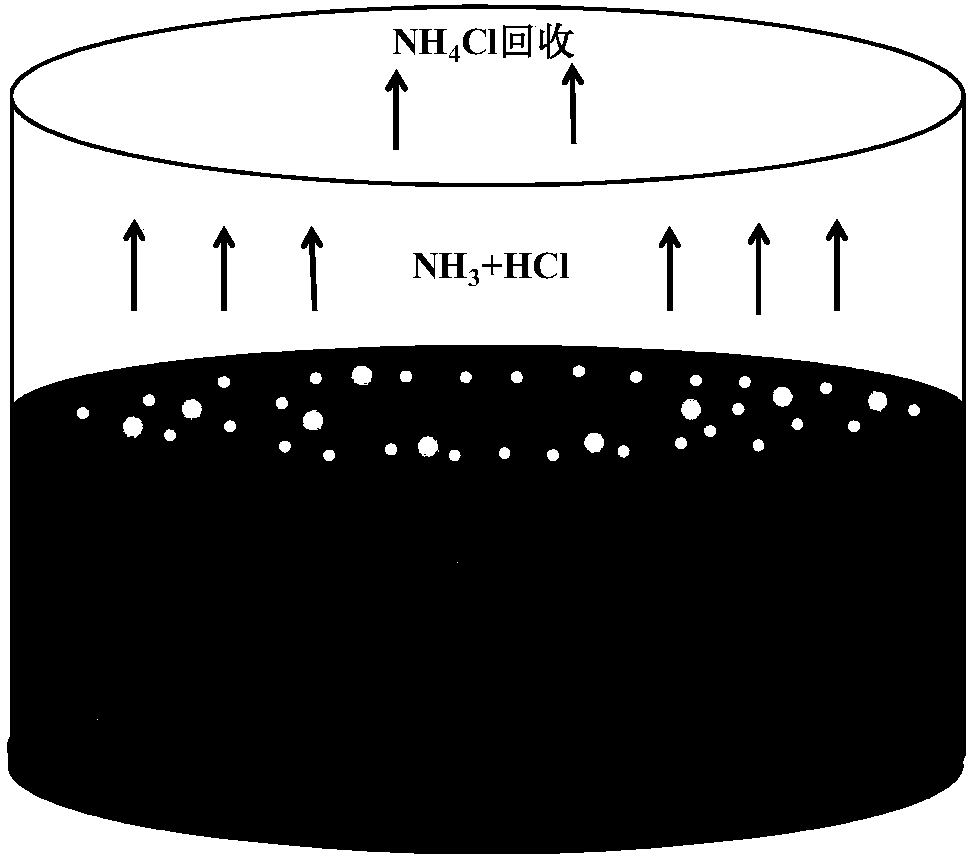
[0040] (4) After placing the corundum crucible in the resistance furnace, fasten the quartz condenser above the crucible to recover the rapidly volatilized NH 4 Cl, causing ammonium chloride to condense on the inner wall of the quartz tube.
[0041] (5) After keeping at 500°C for 5 hours, UO 2 All dissolved. A...
Embodiment 2
[0045] (1) Mix 44.8g LiCl and 55.2g KCl evenly, place in an alumina crucible, place in a vacuum oven, dry at 150°C for more than 72 hours, and set aside.
[0046] (2) 0.26g UO 2 powder with 4g NH 4 The Cl powder was mixed evenly, and spread on top of the LiCl-KCl mixed salt at room temperature.
[0047] (3) Heat the high-temperature resistance furnace to 500°C in an argon-protected glove box, the water and oxygen content of the glove box are less than 5ppm, then place the corundum crucible with the mixture in the resistance furnace, and keep it at a constant temperature of 500°C for reaction.
[0048] (4) After placing the corundum crucible in the resistance furnace, fasten the quartz condenser above the crucible to recover the rapidly volatilized NH 4 Cl.
[0049] (5) After keeping the reaction at 500°C for 5 hours, UO was detected 2 Almost insoluble, just deposited on the bottom of LiCl-KCl molten salt, the dissolution rate is 7% according to ICP-AES measurement.
[005...
Embodiment 3
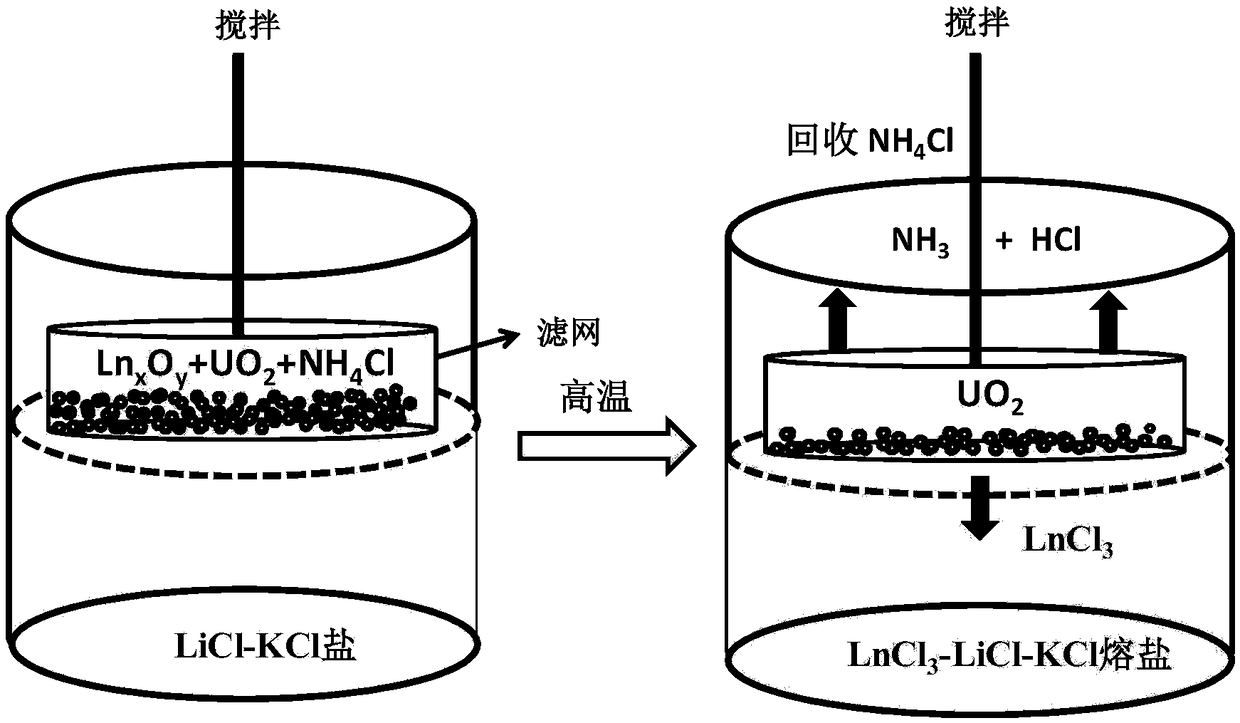
[0053] Through the two types of tests exemplified in Example 1 and Example 2, the inventors found a method based on the addition of ammonium chloride and the isolation of air to separate uranium dioxide and lanthanide oxides. The following is an example of separation (for the process see figure 2 ).
[0054] (1) Mix 44.8g LiCl and 55.2g KCl evenly, place in an alumina crucible, place in a vacuum drying oven, dry at 150°C for more than 72 hours, transfer to an argon-protected glove box for standby, glove box water, The oxygen content is less than 1ppm.
[0055] (2) Add 0.3g UO 2 ,0.5g Sm 2 o 3 ,0.5g Eu 2 o 3 ,0.5g CeO 2 with 10g NH 4 Cl is mixed evenly, and placed in a flat-bottomed container made of a 300-mesh molybdenum filter. The top of the container is tied tightly with molybdenum wire, and the molybdenum wire is drawn out from the hole at the top of the quartz condenser.
[0056] (3) Heat the LiCl-KCl salt prepared in step (1) to 500°C in a glove box. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com