Streptococcus pyogenes HtsA protein vaccine as well as preparation method and application thereof
A Streptococcus pyogenes and protein technology, applied in antibacterial drugs, pharmaceutical formulations, bacterial antigen components, etc., can solve problems such as the preparation of Streptococcus pyogenes vaccines, which have not been seen, achieve significant protection, improve survival rate, and improve immunity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
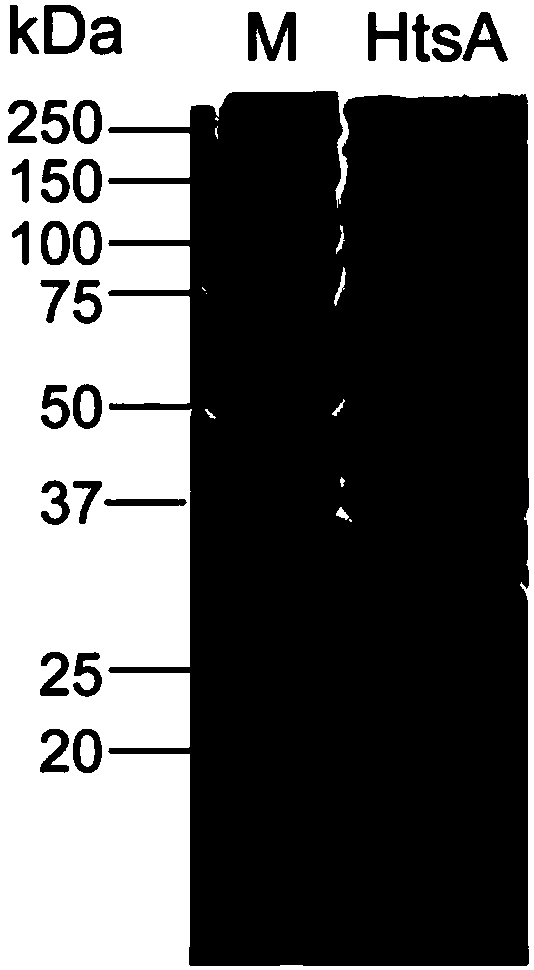
[0042] 1. A method for preparing the recombinant protein of Streptococcus pyogenes HtsA, comprising the steps of:
[0043] (1) Connect the HtsA gene with the signal peptide removed to the pBAD-His vector to obtain the expression vector, and introduce the expression vector into Escherichia coli to obtain the expression strain; inoculate the expression strain in LB medium containing 100 ng / µL ampicillin, Shake culture at 37°C overnight, and continue to expand the culture;
[0044] (2) Proliferate the expression strain to OD 600 When the concentration is 0.6-0.8, add L-arabinose to the culture system to a mass concentration of 0.04%, induce expression for 6 h, collect the cells, and lyse to obtain protein;
[0045] (3) Ni-NTA was used for affinity chromatography to obtain His-HtsA protein; during the affinity chromatography, the column was equilibrated with an equilibrium solution containing 20 mM imidazole. In this example, the use of 20 mM imidazole can effectively reduce the...
Embodiment 2
[0052] Example 2 Detection of immune response effect
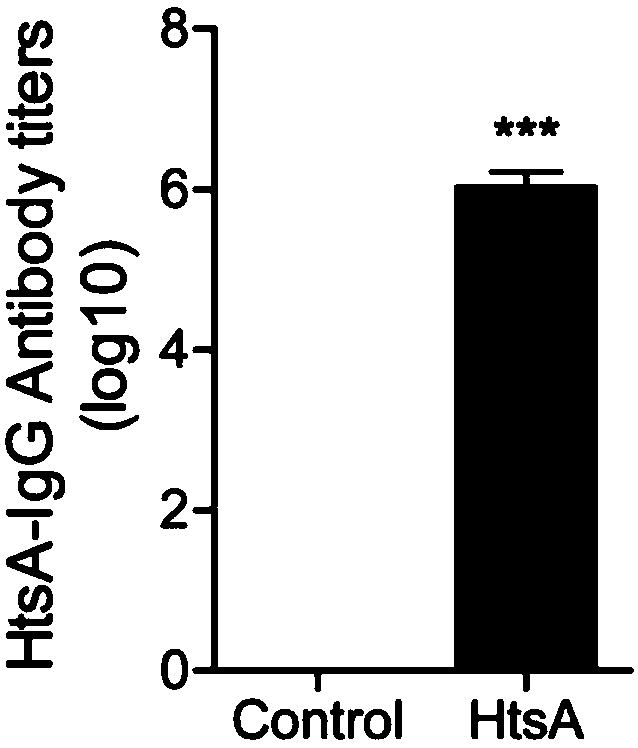
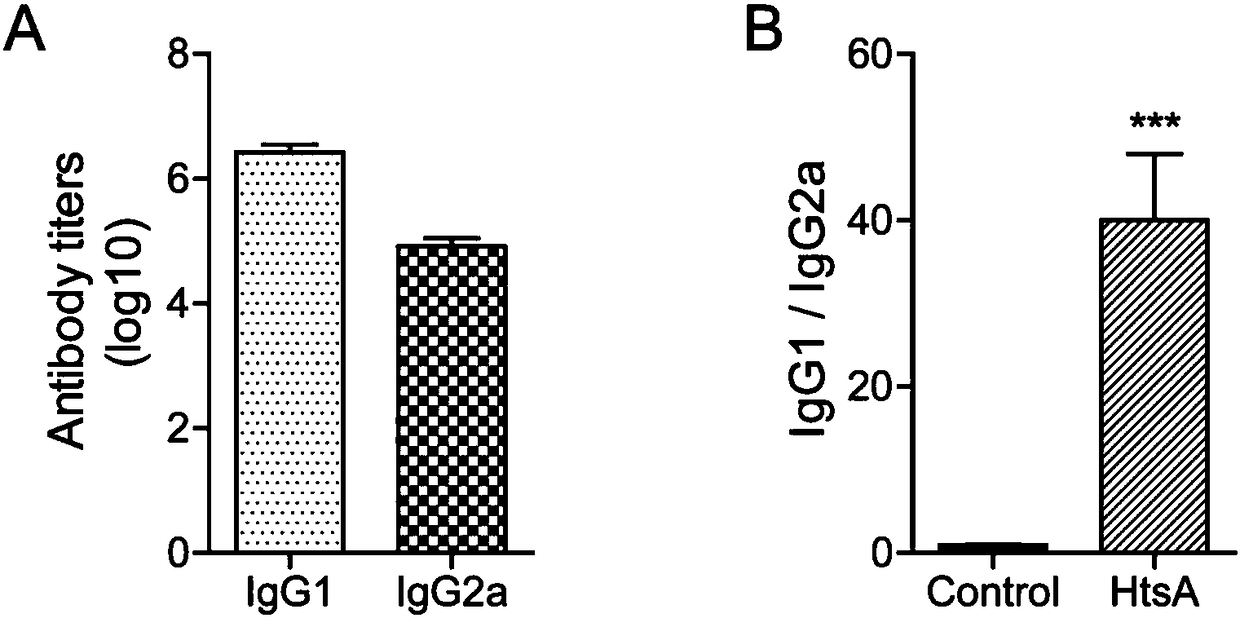
[0053] 1. The mixture of His-HtsA protein and aluminum adjuvant prepared in Example 1 was used to subcutaneously immunize Balb / c mice at multiple sites, and the control group was injected with a mixture of 1×PBS and aluminum adjuvant, 25 μg protein / mouse, each Group 6 mice were immunized three times with an interval of 14 days between each immunization, and the HtsA antibody titer in the mouse serum was detected 7 days after the third immunization. Usually, the immune response caused by protein vaccines is humoral immunity. During humoral immunity, the body produces IgG antibodies in the serum to resist the invasion of pathogens through different mechanisms. Therefore, the antibody titer of IgG in the serum reflects the intensity of the humoral immune response caused by the antigen.
[0054] IgG antibody titers in serum after immune response figure 2 As shown, the results show that after three immunizations, the HtsA an...
Embodiment 3
[0057] In this example, the protective effect of the HtsA recombinant protein vaccine was evaluated through a mouse bacteremia model and a skin infection model.
[0058] 1. In the bacteremia model, inject 2.5×10 7 Streptococcus pyogenes, observed the survival of the mice every day, the results were as follows Figure 4 As shown in A, the survival rate of the mice in the HtsA protein antigen immunized group was significantly higher than that in the control group, with statistical significance.
[0059] 2. In the skin infection model, by subcutaneously inoculating 1×10 7 pyogenes, observed and measured the abscess size of the mice every day, compared with the control group, the abscess of the mice in the HtsA protein antigen immunized group was significantly smaller, and basically recovered on the 7th day after infection ( Figure 4 B ~ 4D shown). The above results show that both the HtsA protein has a protective effect in the mouse bacteremia model and the skin infection mod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com