Application of interleukin-23 neutralizing antibody in preparation of endometriosis medicine
A technology of interleukin and endometrium, which is applied in the field of application of interleukin-23 neutralizing antibody in the preparation of endometriosis drugs, can solve the problem of incompletely clear pathogenesis, poor treatment effect, disease Easy to relapse and other problems, to achieve the effect of reducing size, inhibiting immune escape, and enhancing levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
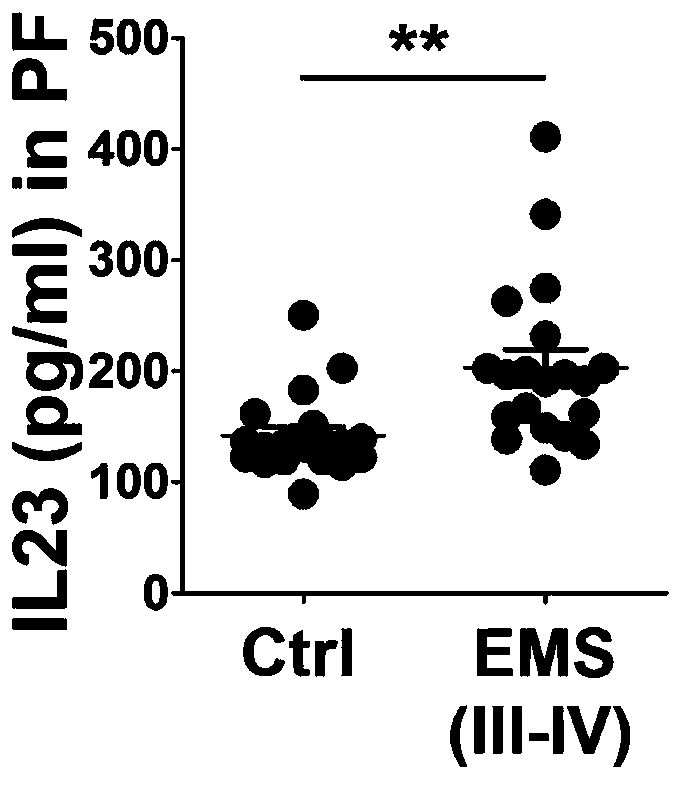
[0023] The level of IL-23 in peritoneal fluid from patients with endometriosis III-IV was analyzed.
[0024]According to the staging standard of endometriosis revised by the American Society for Reproductive Medicine in 1997: stage I (miniature) 1-5 points; stage II (mild) 6-15 points; stage III (medium) 16-40 points; stage IV (Heavy)>40 points. Clinically, patients with obvious symptoms such as dysmenorrhea, chronic pelvic pain, infertility, and requiring drug or surgical treatment are generally III-IV stage, so the research object of the present invention is mainly for III-IV endometriosis patients.
[0025] The collection of peritoneal fluid, endometrium and heterotopic lesions in the control group and EMS group were approved by the Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University School of Medicine and informed consent was obtained from the patients. Peritoneal fluid samples from 20 normal control groups were collected from December 2016 to August ...
Embodiment 2
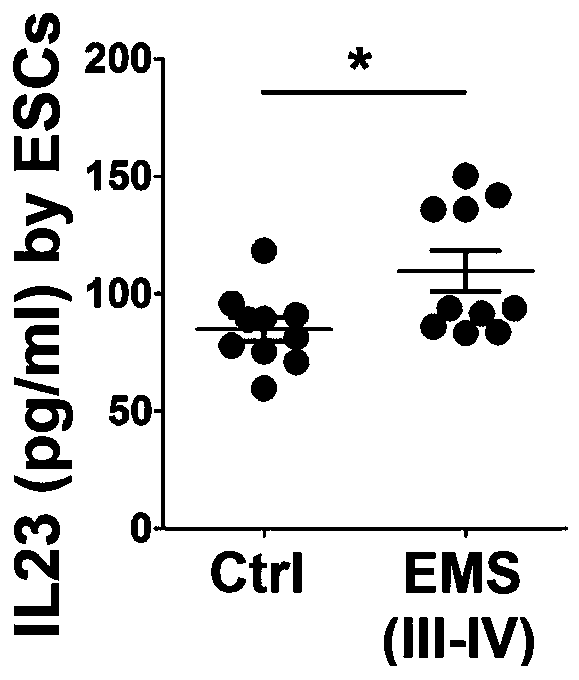
[0028] The level of IL-23 secretion in ESCs of ectopic foci was analyzed.
[0029] The 10 samples of the control group were obtained from patients who underwent curettage for "menstrual disorders" in the Drum Tower Hospital Affiliated to Nanjing University School of Medicine from December 2016 to August 2017. 5 cases; 10 cases of ectopic lesions were obtained from patients with III-IV endometriosis who underwent laparoscopic ovarian endometrioid cystectomy at the same time, and the nature of ovarian endometrioid cysts was confirmed by pathology after surgery. The two groups of patients were between 22 and 40 years old, had regular menstrual cycles, biphasic basal body temperature, and no other medical or gynecological complications. They did not take hormone drugs 3 months before the operation.
[0030] During the operation, the tissues of the control group or ectopic focus were collected, placed in DMEM-F12 culture medium under aseptic conditions, and stored on ice. ESC (endo...
Embodiment 3
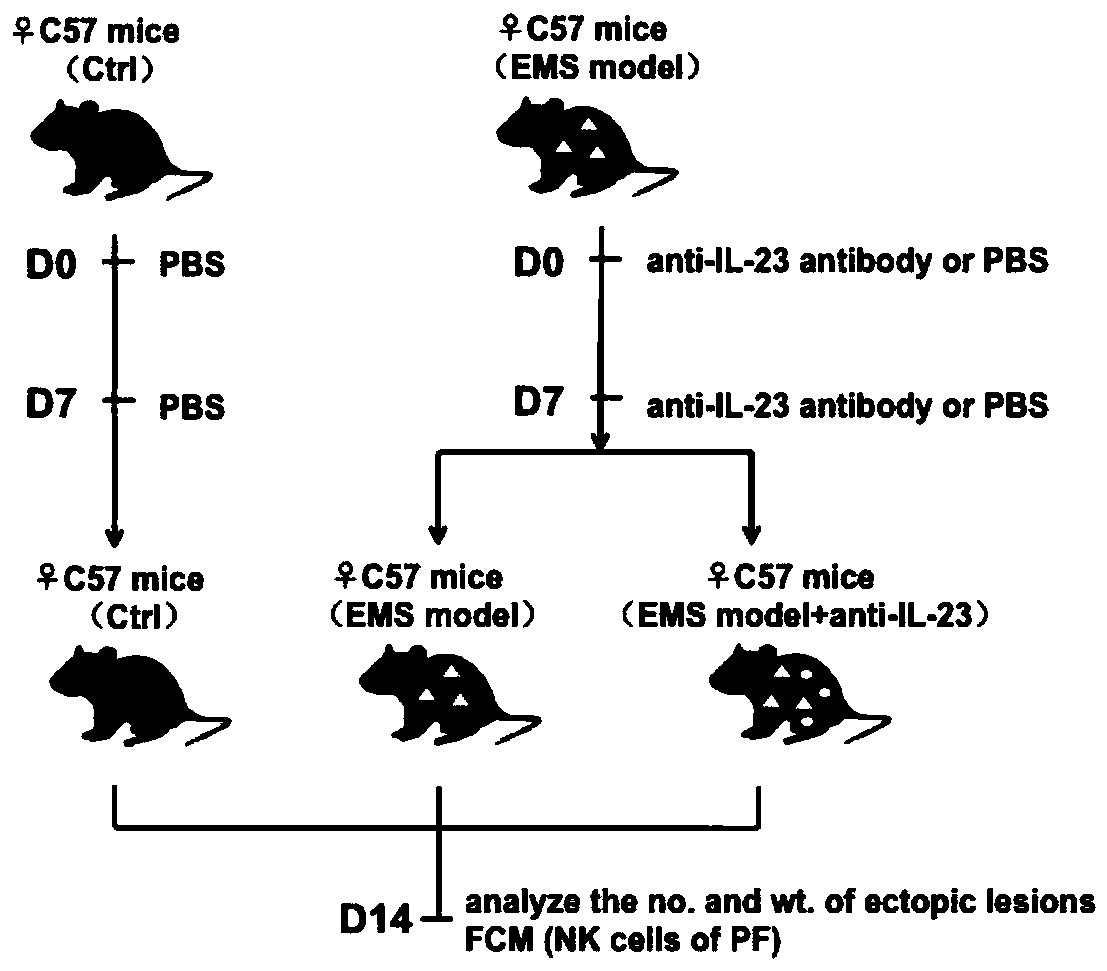
[0033] Construction of EMS mouse model.
[0034] Specifically: select healthy 7-week-old female C57B / L6 mice (Shanghai Shrek Experimental Animal Co., Ltd.), induce with intramuscular injection of estrogen once a week before the operation, take out the uterus of the donor mouse, and cut it into pieces <1mm Tissue fragments, the shredded uterine tissue was injected into recipient mice (uterine fragments of one donor mouse were divided into two and injected into two test mice). The day of modeling was D0, and intraperitoneal injection of IL-23 neutralizing antibody (anti-IL-23, purchased from R&D Company) or control PBS (pH =7.2±0.1).
[0035] Wherein, the IL-23 neutralizing antibody injected above is mouse-derived IL-23p19 antibody, prepared according to the instructions with phosphate buffered saline PBS (pH=7.2±0.1) containing 10% (V / V) fetal bovine serum. Store at an initial concentration of 1 mg / ml, and continue diluting to the working concentration with phosphate buffer s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com