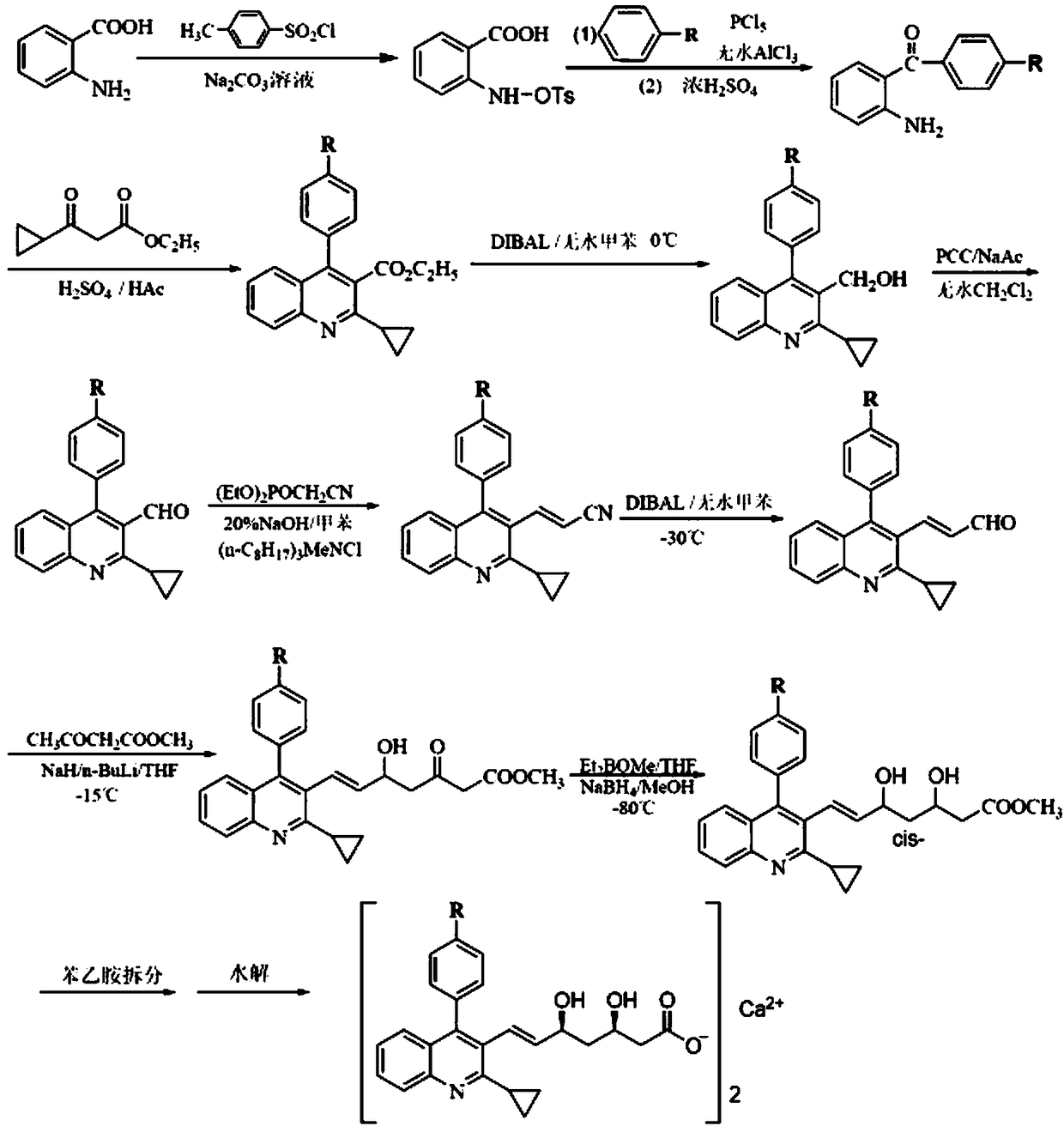
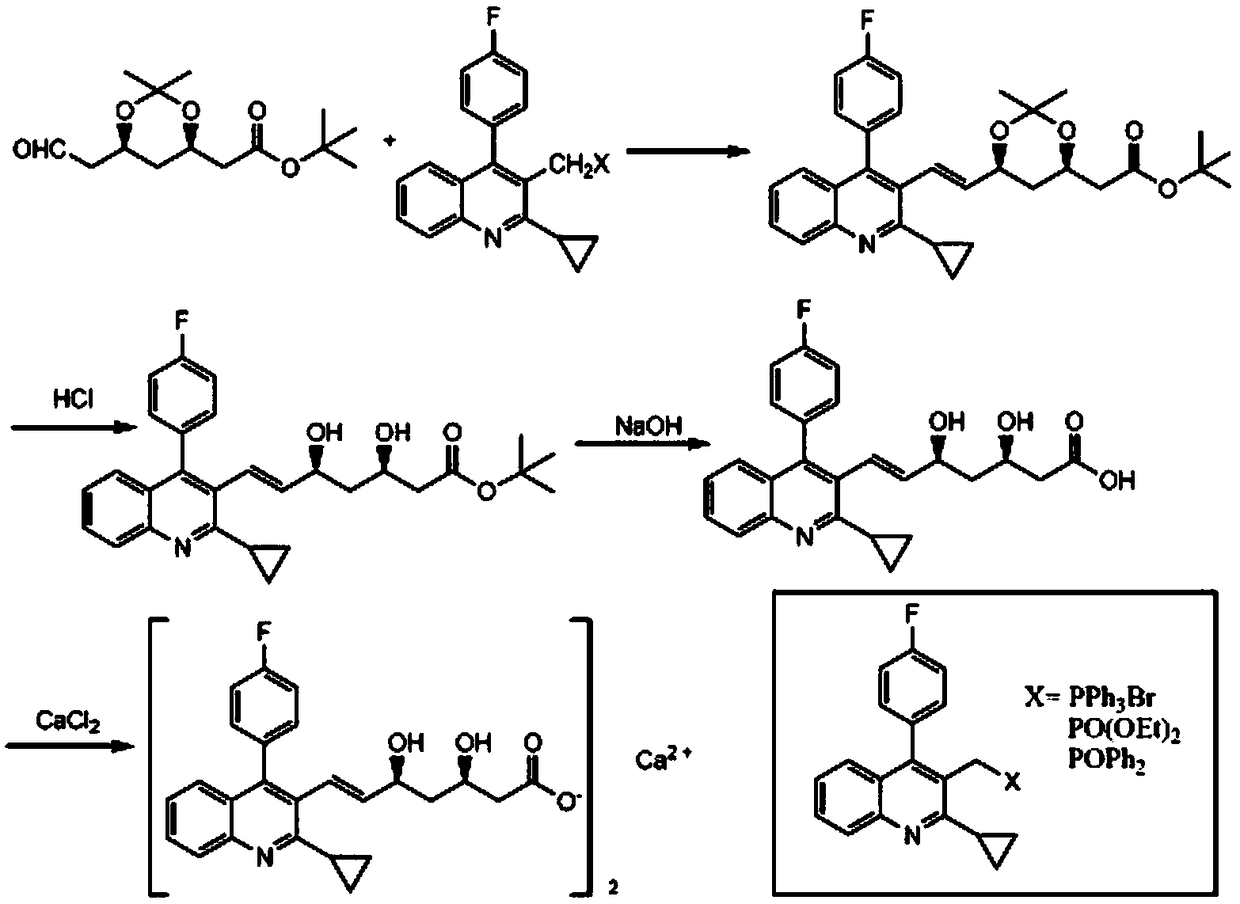
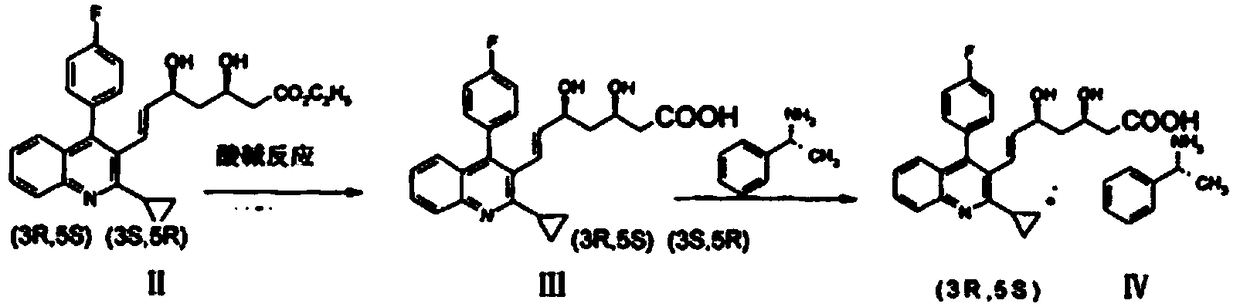
Pitavastatin (III) refining method
A technique of refining pitavastatin, which is applied in the field of drug synthesis, can solve the problems of low yield, difficult purification, and unsuitability for large-scale industrial production, and achieve the effects of increasing yield, reducing solvent consumption, and reducing heating time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The refining method of pitavastatin with high optical purity, which is suitable for industrial production, has few impurities and high yield, is carried out according to the following steps:
[0037] Step 1: Prepare a mixed solvent of water, DMF and methyl isobutyl ketone, the weight ratio of water, DMF and methyl isobutyl ketone is 2:10:180.
[0038] Step 2: The crude product of pitavastatin (Ⅲ) (8.4kg, purity: 78.9%, epimer: 0.72%, Z-isomer: 13.5%, its HPLC figure is shown in Figure 4 shown) was added to the mixed solvent to dissolve and mechanically stirred. The weight of the mixed solvent was 21kg, which was 2.5 times the weight of the crude product of pitavastatin (Ⅲ). D-(+)-α-methylbenzylamine (2.5kg), continue to maintain stirring, solids begin to precipitate, and the suspension is maintained at 20-30°C and stirred for 2 hours;
[0039] Step 3: filter the suspension, drain as far as possible to no drip, then wash the filter cake with a small amount of mixed sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com