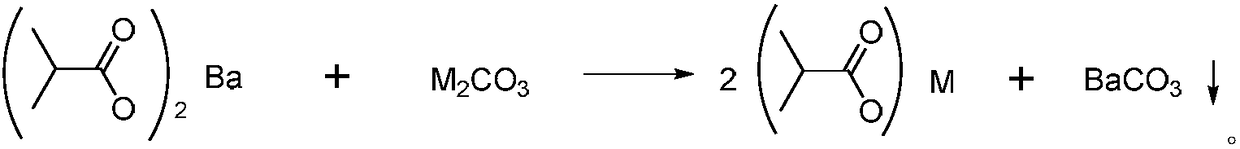
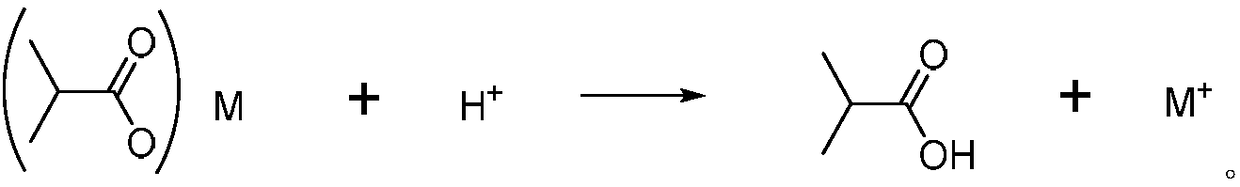
Method for recovering barium in wastewater containing barium isobutyric acid through carbonate precipitation method
A technology of carbonate precipitation and barium isobutyrate, which is applied in barium carbonate, calcium carbonate/strontium/barium, chemical instruments and methods, etc., can solve problems such as difficult processing, unreported heavy metal ion recovery methods, etc., and achieve COD value low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, a kind of carbonate precipitation method reclaims the method containing barium in barium isobutyrate waste water, carries out following steps successively:
[0035] 1), 20g (0.19mol) of sodium carbonate was dissolved in water to prepare a saturated sodium carbonate solution, which was added dropwise at 20°C to 100g of barium isobutyrate waste water (containing 0.16mol of barium ions) with a mass fraction of 50%. During the addition process, the temperature is controlled to be ≤30°C. After the dropwise addition is completed, the measured pH is about 10;
[0036] 2), filtering (filtering immediately after the dropwise addition) the reaction solution obtained in step 1) to obtain the filtrate and filter cake respectively; The ion recovery rate is 100%;
[0037] In the filtrate (filtered gained aqueous phase), adding mass fraction is 128g (containing HCl 0.35mol) of dilute hydrochloric acid (HCl 0.35mol) of 10%, this moment, pH is about 2; 121 ° C fraction),...
Embodiment 2
[0039] Embodiment 2, a kind of carbonate precipitation method reclaims the method containing barium in barium isobutyrate waste water, carries out following steps successively:
[0040] 1), potassium carbonate 26.2g (0.19mol) is dissolved in water and is mixed with saturated potassium carbonate solution, and is added dropwise at 20 ℃ in the barium isobutyrate waste water 100g (containing barium ion 0.16mol) that mass fraction is 50%, During the dropping process, the temperature is controlled to be ≤30°C. After the addition is completed, the measured pH is about 10;
[0041] 2), filter (filter immediately after dropwise addition) step 1) gained reaction solution, obtain filtrate and filter cake respectively; Obtain barium carbonate 31g (0.16mol) after filter cake drying, barium ion recovery rate 100%;
[0042] In filtrate, adding mass fraction is 128g (containing HCl 0.35mol) of dilute hydrochloric acid (HCl 0.35mol) of 10%, and now pH is about 2; Rectification under reduced pr...
Embodiment 3
[0044] Embodiment 3, a kind of carbonate precipitation method reclaims the method containing barium in barium isobutyrate waste water, carries out following steps successively:
[0045] 1), ammonium carbonate 18g (0.19mol) is dissolved in water and is mixed with saturated ammonium carbonate solution, is added dropwise in the barium isobutyrate waste water 100g (containing barium ion 0.16mol) that mass fraction is 50% at 20 ℃, drop During the addition process, the temperature is controlled to be ≤30°C. After the addition is completed, the measured pH is about 10;
[0046] 2), filter (filter immediately after dropwise addition) step 1) gained reaction solution, obtain filtrate and filter cake respectively; Obtain barium carbonate 31g (0.16mol) after filter cake drying, barium ion recovery rate 100%;
[0047] In filtrate, adding mass fraction is 128g (containing HCl 0.35mol) of dilute hydrochloric acid (HCl 0.35mol) of 10%, and now pH is about 2; Rectification under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com