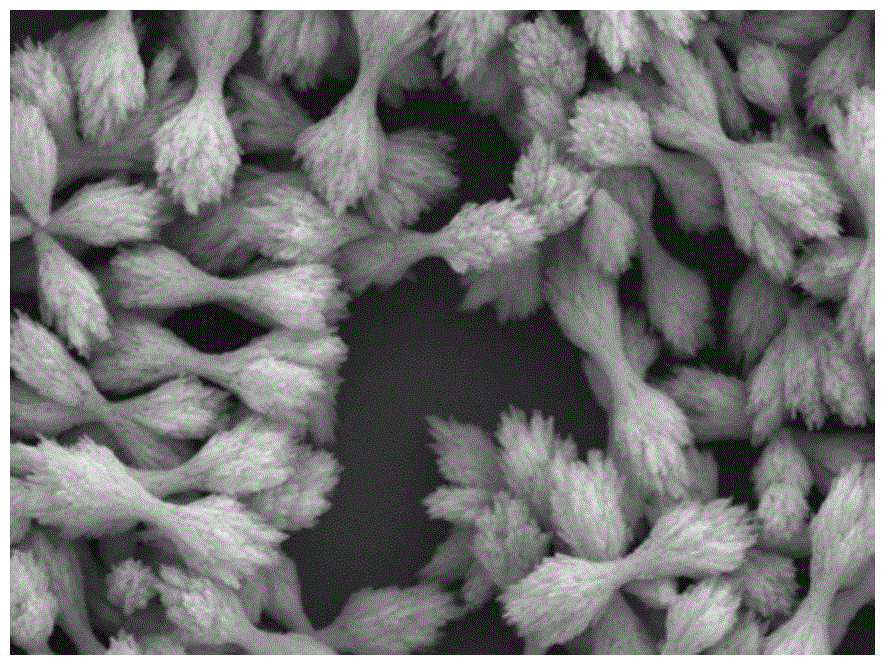
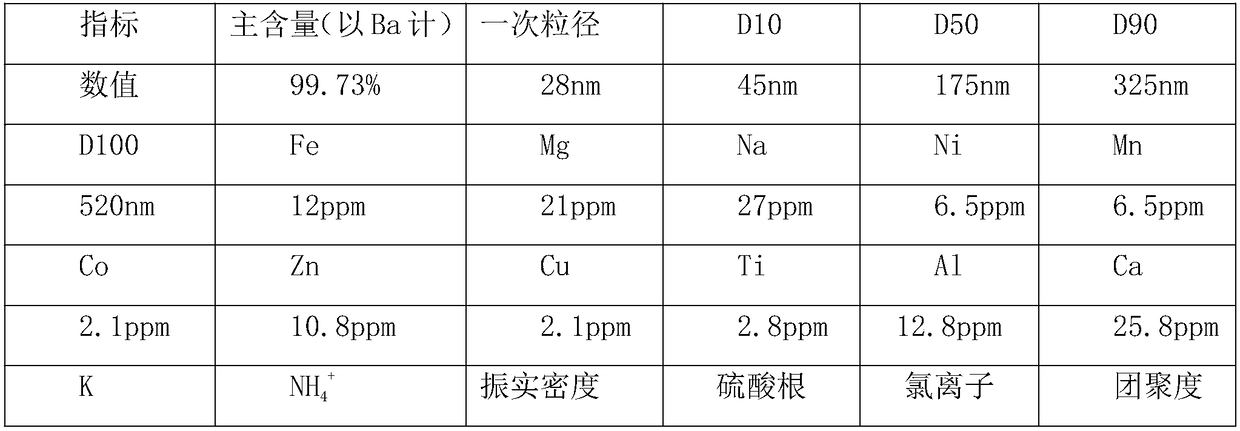
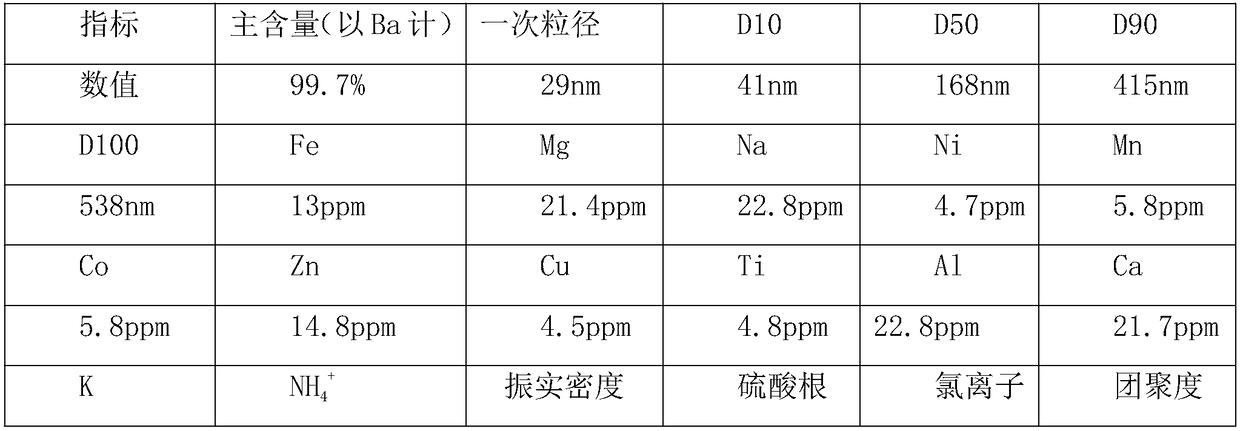
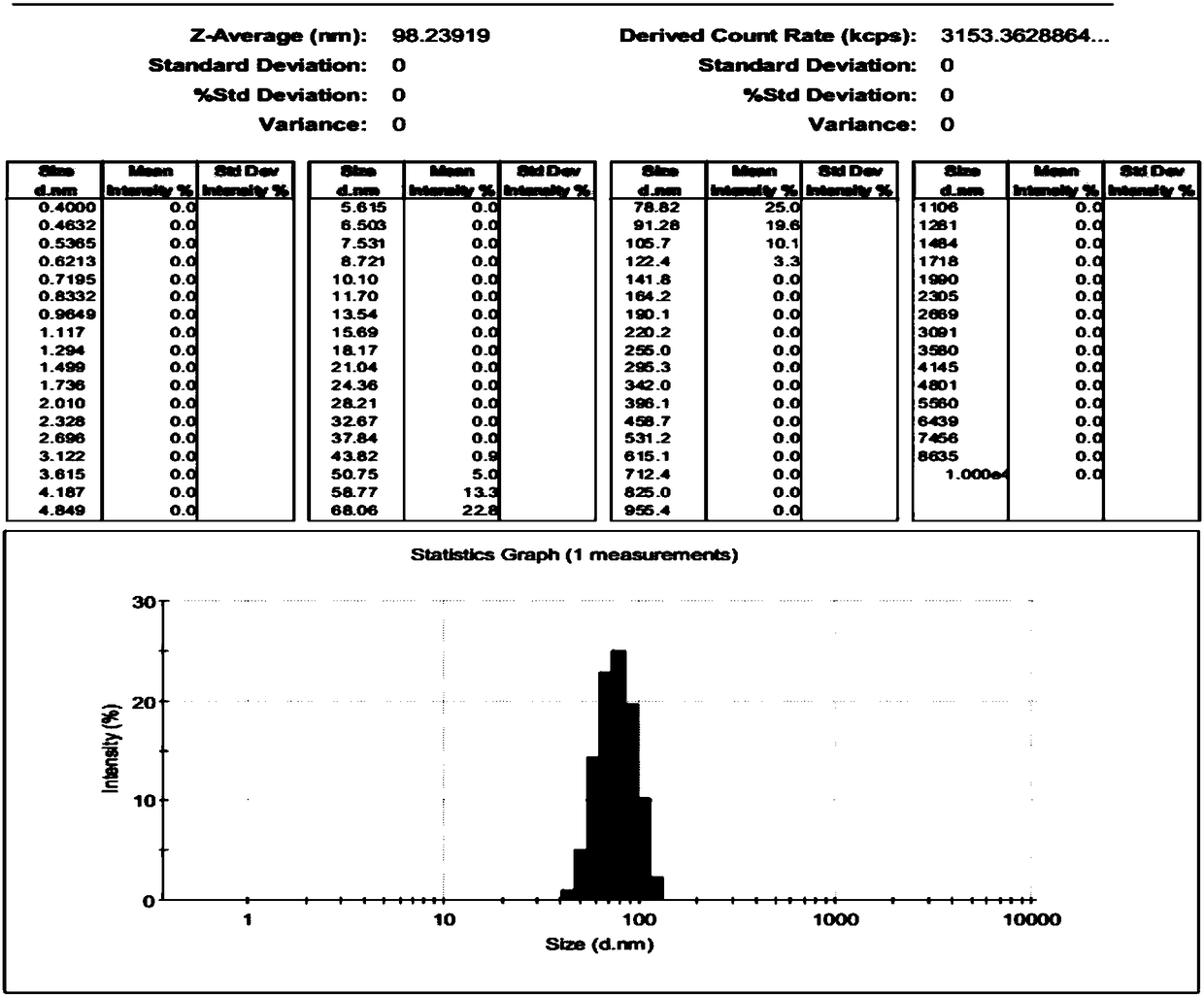
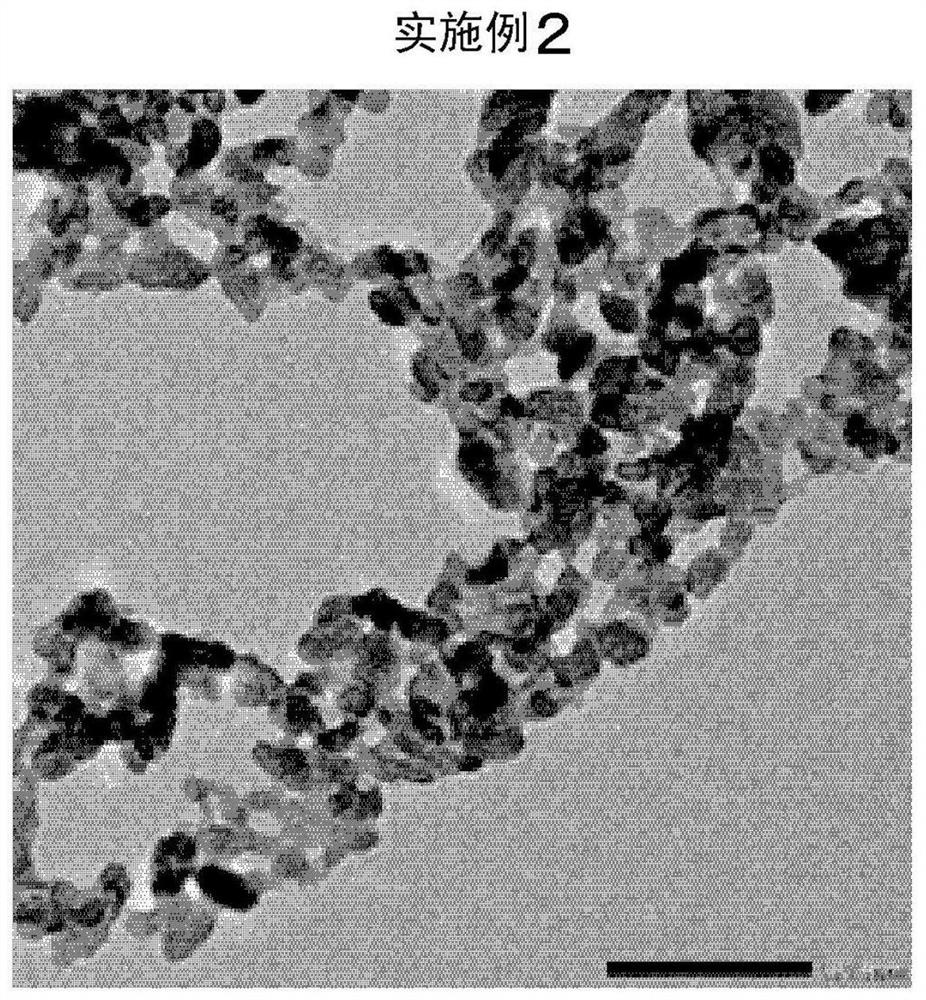
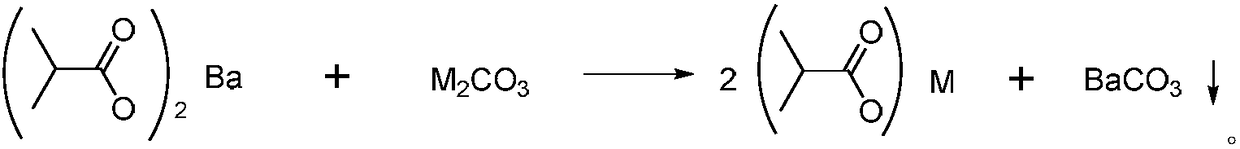
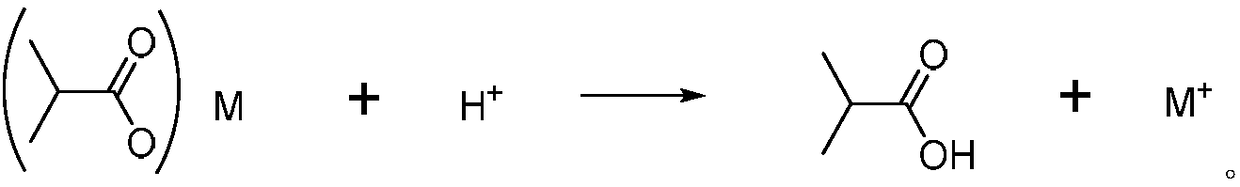
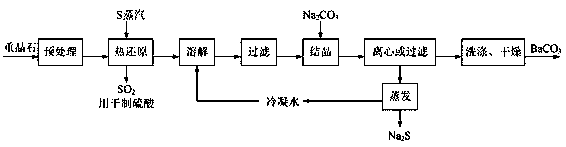
Patents
Literature
59results about "Barium carbonates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
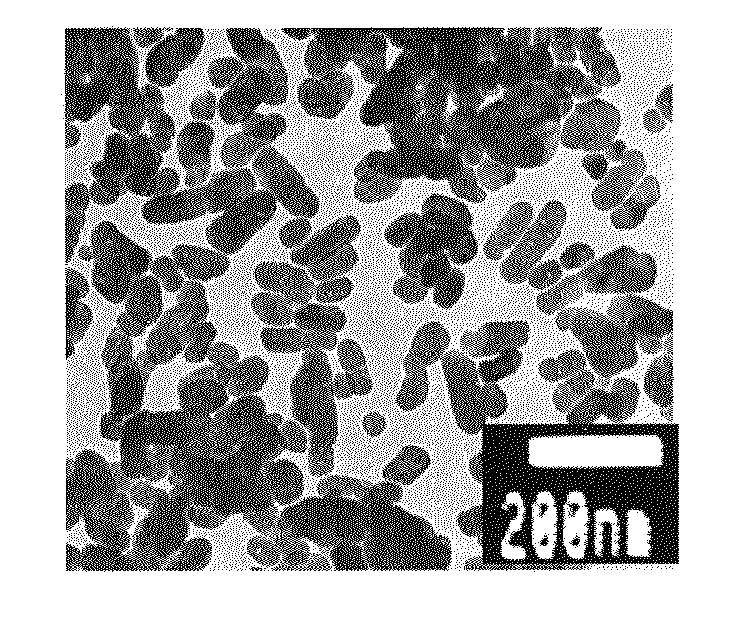
Process for preparing micron/nano size inorganic particles
InactiveUS20050218540A1Easy to controlHigh purityMaterial nanotechnologyBarium carbonatesSulfideChemistry
The present invention discloses methods for making micron / nano sized (2 nm to 5 μm) particles of various inorganic materials such as mineral / oxides / sulphides / metals / ceramics using aqueous foam, Aqueous foams of various anionic, cationic, non-ionic surfactant, casein proteins and their mixtures has been used for the preparation of suitable inorganic materials growth. Large scale synthesis of advanced inorganic materials such as various ceramics, minerals, oxides, sulphides and metal micron / nanoparticles of controlled shape and size can be obtained by mixing appropriate metal ions with the suitable cationic / anionic / non-ionic / casein protein / their mixtures, which is bubbled by air to form aqueous foams and thereafter their reduction / reaction to form the final product.
Owner:COUNCIL OF SCI & IND RES
Barium titanate powder and preparation method thereof
InactiveCN1362385ALow dielectric propertiesAlkaline earth titanatesBarium carbonatesCapacitanceBarium titanate
Owner:MURATA MFG CO LTD
Barium carbonate particle powder, production method thereof, and production method of perovskite barium titanate
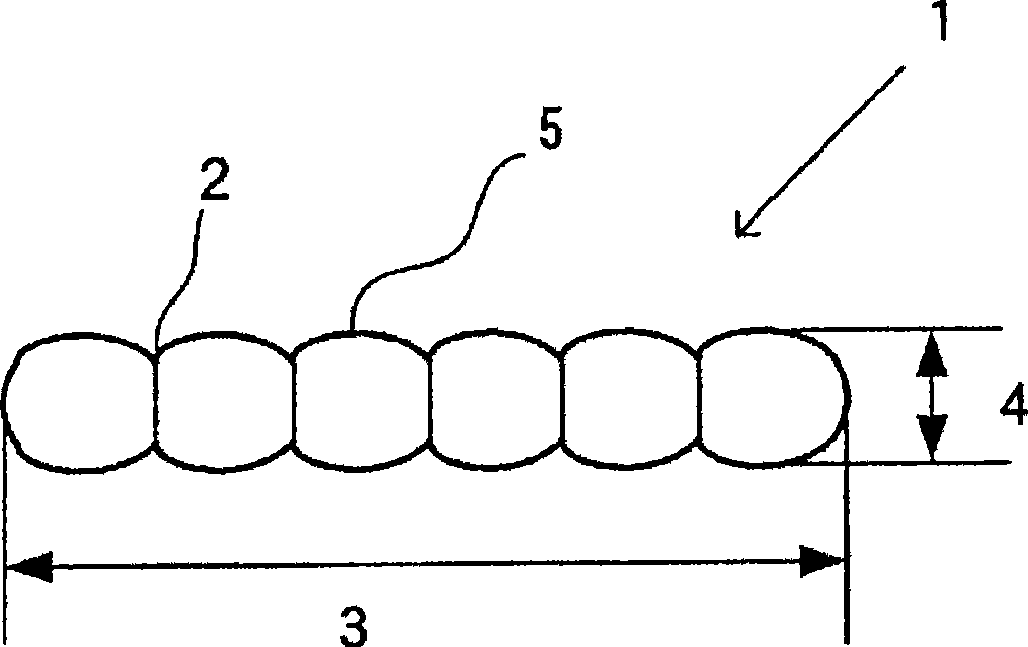
ActiveCN101428838ASmall aspect ratioGood dispersionAlkaline earth titanatesBarium carbonatesBarium titanateSlurry
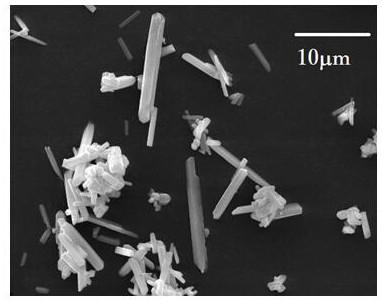
A rod-like barium carbonate particle powder excellent in dispersibility and small in aspect ratio and a production method thereof are provided. The rod-like barium carbonate particle powder includes rod-like particles having one or two or more constricted portions on the particle surface and the aspect ratio of the rod-like particles is 1.5 to 4.5. The BET specific surface area of the rod-like particle is 15 m 2 / g or more. The constricted portions are disposed in the direction perpendicular to or inclined from the long axis direction of the rod-like particles. The production method of the rod-like barium carbonate particle powder includes a step of heat treating an aqueous slurry containing an acicular barium carbonate particle powder at 50 DEG C or higher. Also claimed are a method for producing perovskite barium titanate making use of the barium carbonate particle powder and a barium source comprising the barium carbonate powder.
Owner:NIPPON CHECMICAL IND CO LTD
Preparation method of barium carbonate and product prepared by same
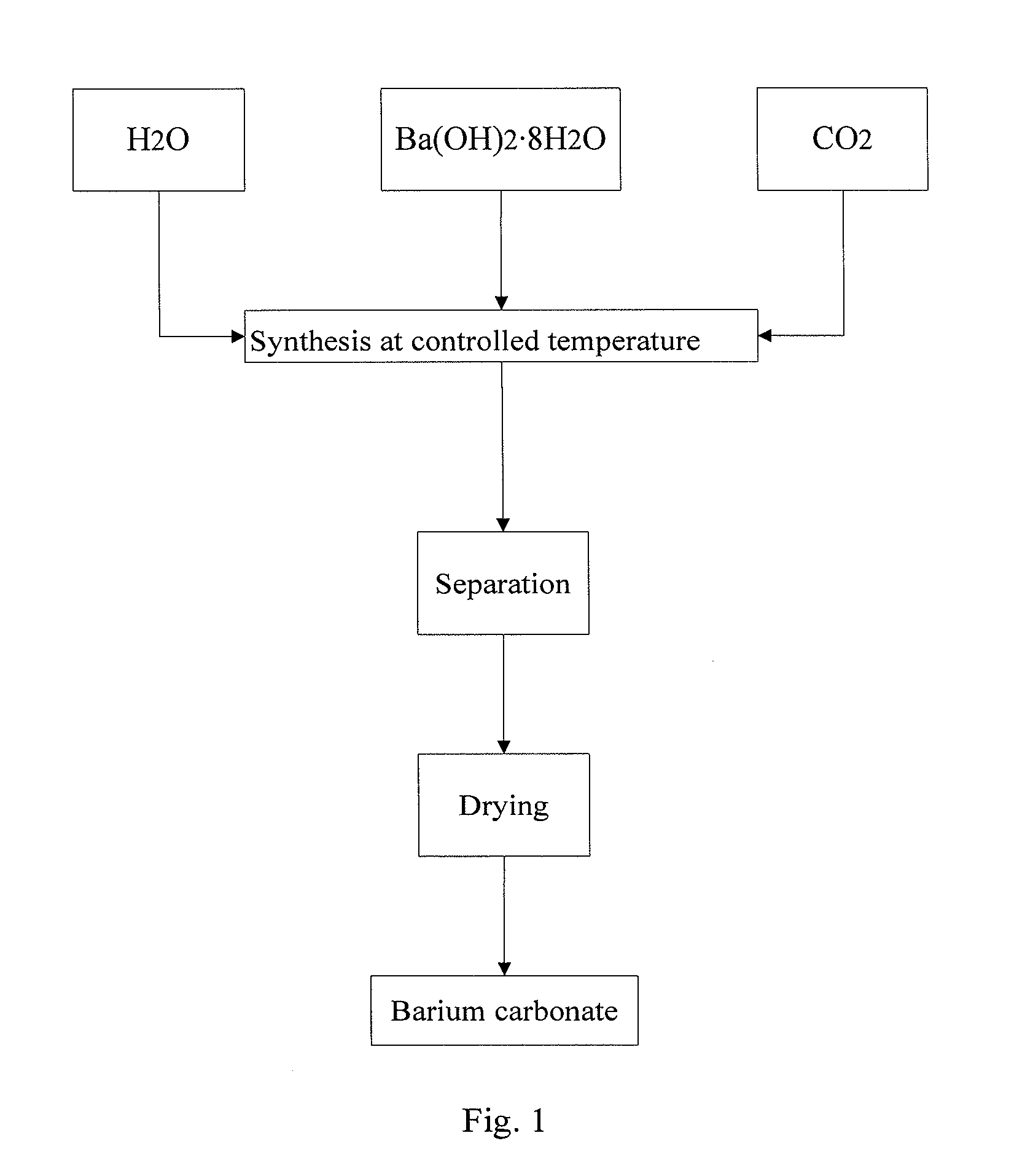
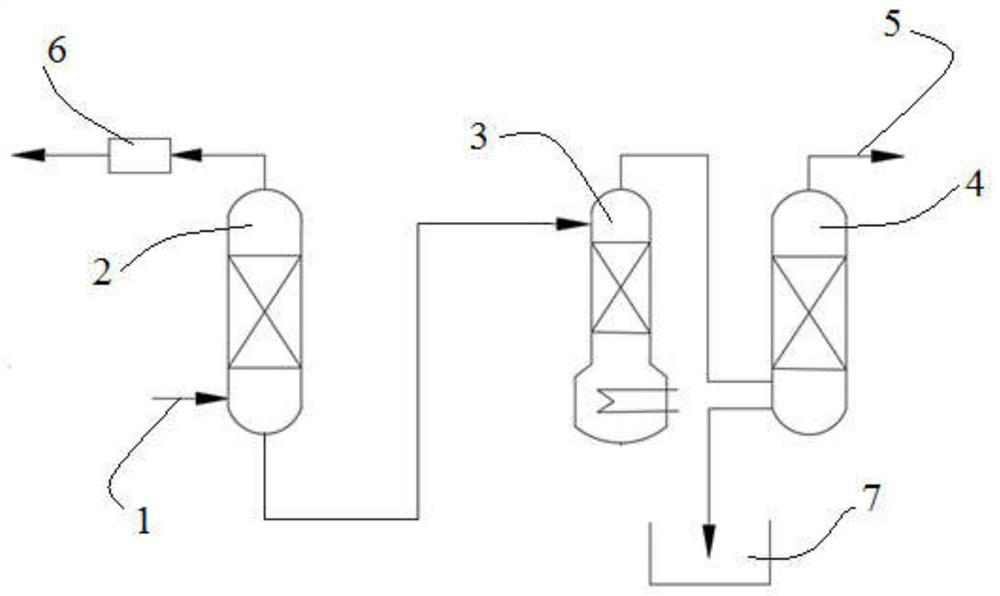
ActiveCN102674426ANo longer affects sintering temperatureImprove performanceBarium carbonatesThin material handlingHydration reactionIce water
The invention relates to a preparation method of barium carbonate and a barium carbonate product prepared by the same. The preparation method of the barium carbonate comprises the following steps: (1) adding barium hydroxide octahydrate crystals into an ice water bath, and mixing, wherein the temperature is controlled at 0-5 DEG C; (2) introducing carbon dioxide into the mixture obtained in the step (1) to react until the pH value of the reaction solution is 6.0-7.0; and (3) carrying out solid-liquid separation on the solid-liquid mixture obtained in the step (2), and drying the solid to obtain the large-specific-area high-purity barium carbonate product. The preparation method provided by the invention solves the problem of incompatible low strontium and large specific area in the existing preparation method, so that the barium carbonate product is no longer influenced by the sintering temperature, thereby enhancing the performance of the ceramic material.
Owner:SHENZHEN MODERN SKY TECH CO LTD
Method for improving the recovery of cesium-131 from barium carbonate
ActiveUS20070212285A1Promote recoveryBarium carbonatesTransuranic element compoundsMedicineBrachytherapy
The present invention provides a method for improving the recovery of cesium-131 (Cs-131) from barium (Ba) carbonate. Uses of the Cs-131 purified by the method include cancer research and treatment, such as for the use in brachytherapy. Cesium-131 is particularly useful in the treatment of faster growing tumors.
Owner:ISORAY MEDICAL INC
Method of manufacturing multilayer ceramic electronic component and multilayer ceramic electronic component
ActiveUS20200111616A1Improve reliabilityIncrease capacitanceBarium carbonatesFixed capacitor electrodesDielectric layerSlurry
A method of manufacturing a multilayer ceramic electronic component includes: preparing a dielectric magnetic composition including base material powder particles including BaTi2O5 or (Ba(1-x)Cax)Ti2O5 (0≤x<0.1), the base material powder particles having surfaces coated with one or more of Mg, Mn, V, Ba, Si, Al and a rare earth metal; preparing ceramic green sheets using dielectric slurry including the dielectric magnetic composition; applying an internal electrode paste to the ceramic green sheets; preparing a green sheet laminate by stacking the ceramic green sheets to which the internal electrode paste is applied; and preparing a ceramic body including dielectric layers and a plurality of first and second internal electrodes arranged to face each other with each of the dielectric layers interposed therebetween by sintering the green sheet laminate.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Barium slag carbon/hydrogen cooperative reduction thermal molten-salt slag-free production process
InactiveCN110937619AImprove leaching utilizationReasonable investmentBarium carbonatesCalcium/strontium/barium chloridesAmmonium sulphideSlag
The invention relates to a barium slag carbon / hydrogen cooperative reduction thermal molten-salt slag-free production process, comprising: (1) heating and roasting barium slag in a rotary kiln by means of glass kiln tail gas, and feeding hydrogen for reduction by controlling material temperature to be 600-1250 DEG C, wherein the hydrogen serves as reductive gas for the barium slag in the rotary kiln and the barium slag is reduced into a thermal material; (2) adding NH4Cl water solution to the upper part of the molten salt reaction tank, and feeding the thermal material into the NH4Cl water solution through the upper part of the molten salt reaction tank to perform a reaction, wherein the temperature of the NH4Cl water solution is controlled to 60-115 DEG C, and a chlorate solid-liquid mixture is obtained and ammonia vapor and ammonium sulfide vapor are generated after the reaction; (3) discharging the reaction products from the bottom of the reaction tank into a solid-liquid separationdevice for solid-liquid separation. By means of the method, effective utilization of the barium slag is achieved, and whole-element recovery of the barium slag is completed at low cost and high efficiency. The method can achieve zero-emission of solid and liquid and very-low CO2 emission, even zero-emission of CO2, in the whole production process.
Owner:胡长春
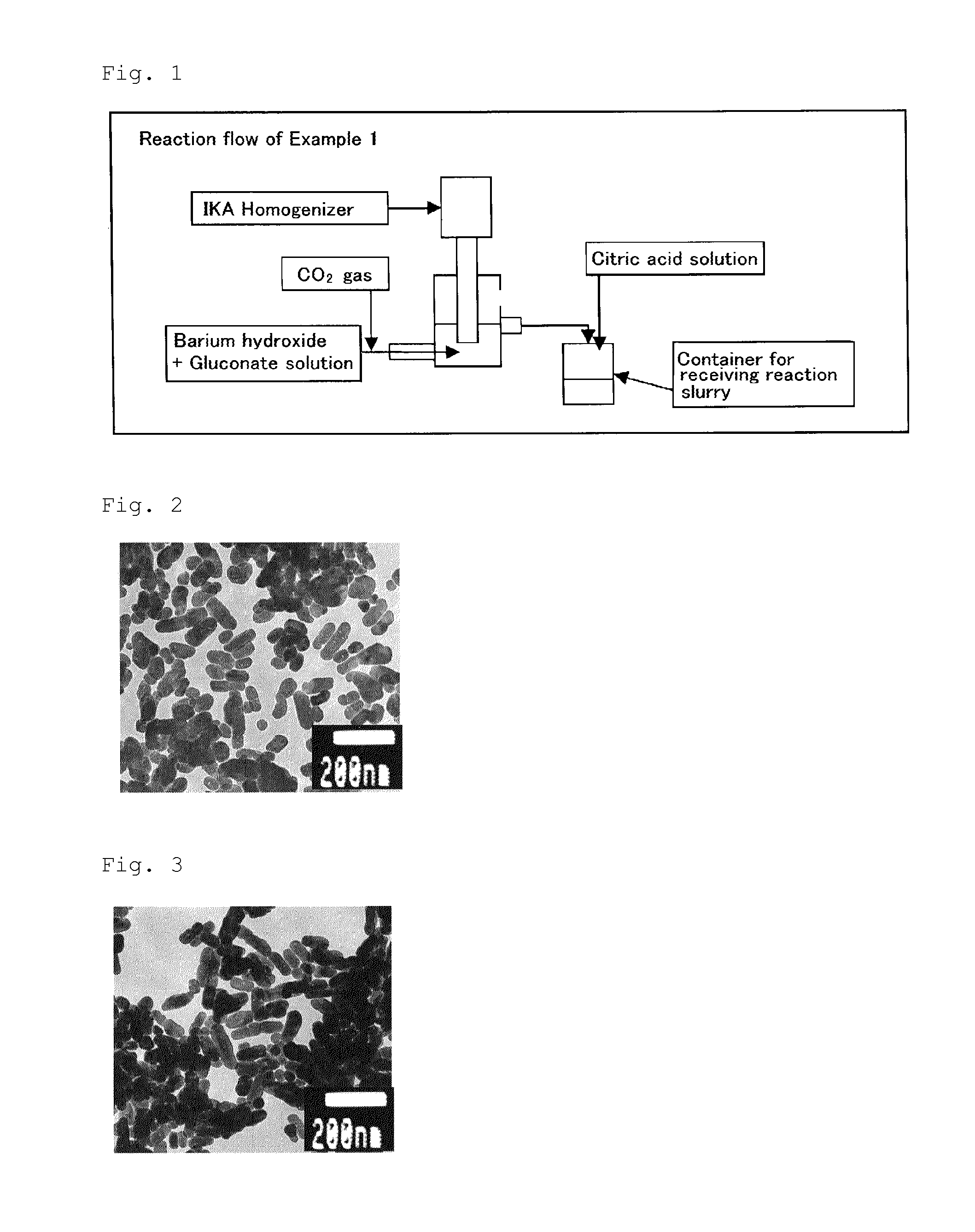
Generally spherical barium carbonate particles, and method for producing generally spherical barium carbonate particles
ActiveUS20120045381A1Uniform shapeUniform sizePigmenting treatmentBarium carbonatesParticulatesAdditive ingredient
The present invention has an object to provide a method for producing particulate barium carbonate having desired properties such as high purity, fineness, and has a spherical shape. The present invention relates to a method of producing substantially spherical barium carbonate, including (A) mixing, in an aqueous medium, a barium compound with at least one first ingredient selected from the group consisting of gluconic acid or salts thereof, gluconolactone, glucoheptonic acid or salts thereof, and glucoheptonolactone, to prepare a mixture; and (B) reacting the barium compound with carbon dioxide or a water-soluble carbonate in the mixture, to produce substantially spherical barium carbonate.
Owner:SAKAI CHEM IND CO LTD
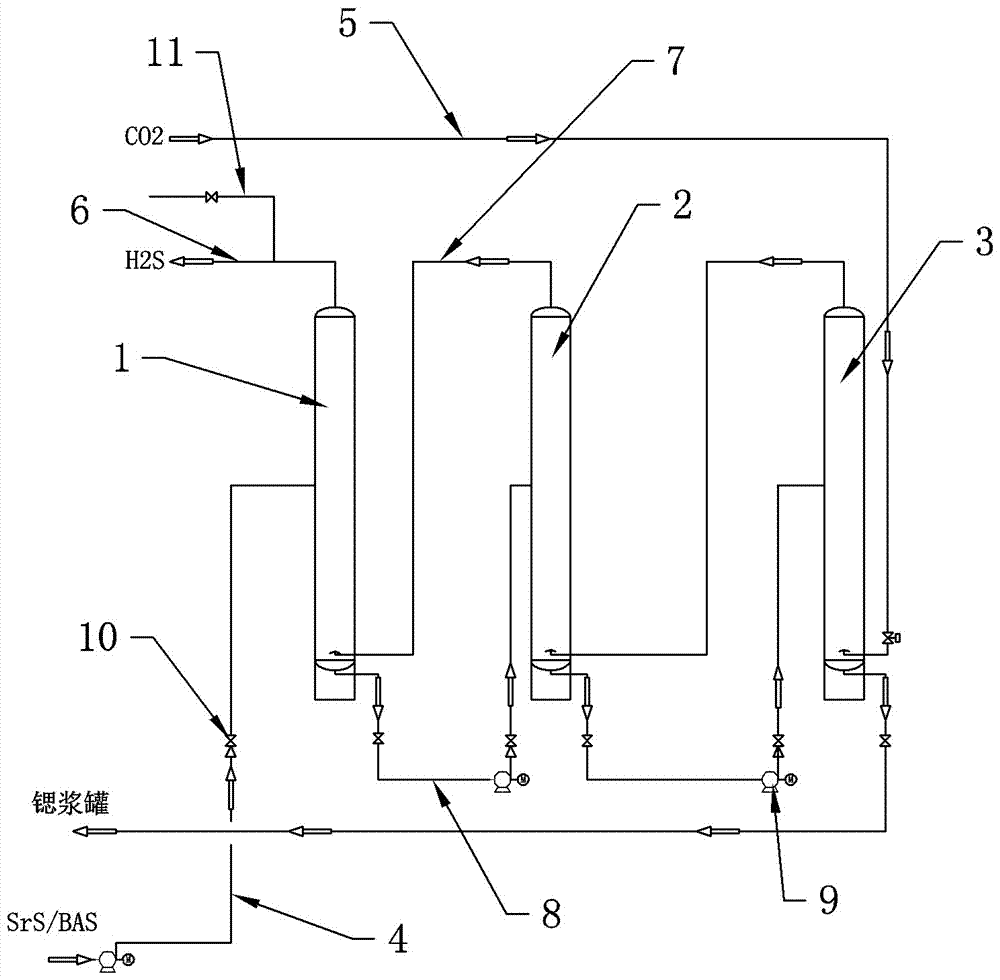
Process for producing strontium carbonate and barium carbonate without continuous carbonization of hydrogen sulfide gas holder
InactiveCN107140670ASubsequent production process is stableBarium carbonatesStrontium carbonatesStrontium carbonateStrontium sulfide
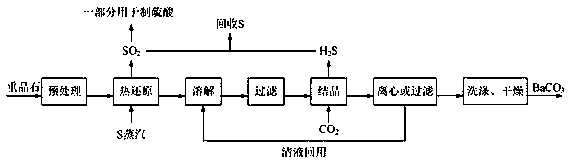
The application of the present invention discloses a process for continuously carbonizing strontium carbonate and barium carbonate without hydrogen sulfide gas tank, which includes the following process steps: the first step is to pass the strontium sulfide or barium sulfide liquid through the liquid feed pipe first from the first carbonization tower The upper part is sprayed down; the second step is to adjust the flow rate to maintain a stable liquid level in the first carbonization tower; the third step is to adjust the flow rate to maintain a stable liquid level in the second carbonization tower, and so on; The fourth step is to completely react carbon dioxide with strontium sulfide or barium sulfide solution, and the hydrogen sulfide gas produced is discharged from the exhaust pipe at the top of the first carbonization tower into the subsequent production process; the fifth step is to discharge strontium sulfide or barium strontium sulfide slurry , and then adjust the flow rate to maintain a stable liquid level in the last carbonization tower; the sixth step is to start the car and start continuous production according to the above process. By adopting the process of the invention, the hydrogen sulfide gas tank can be eliminated, and at the same time, the production stability of the follow-up process using hydrogen sulfide as a raw material can be guaranteed.
Owner:CHONGQING KINGLONG FINE STRONTIUM CHEM
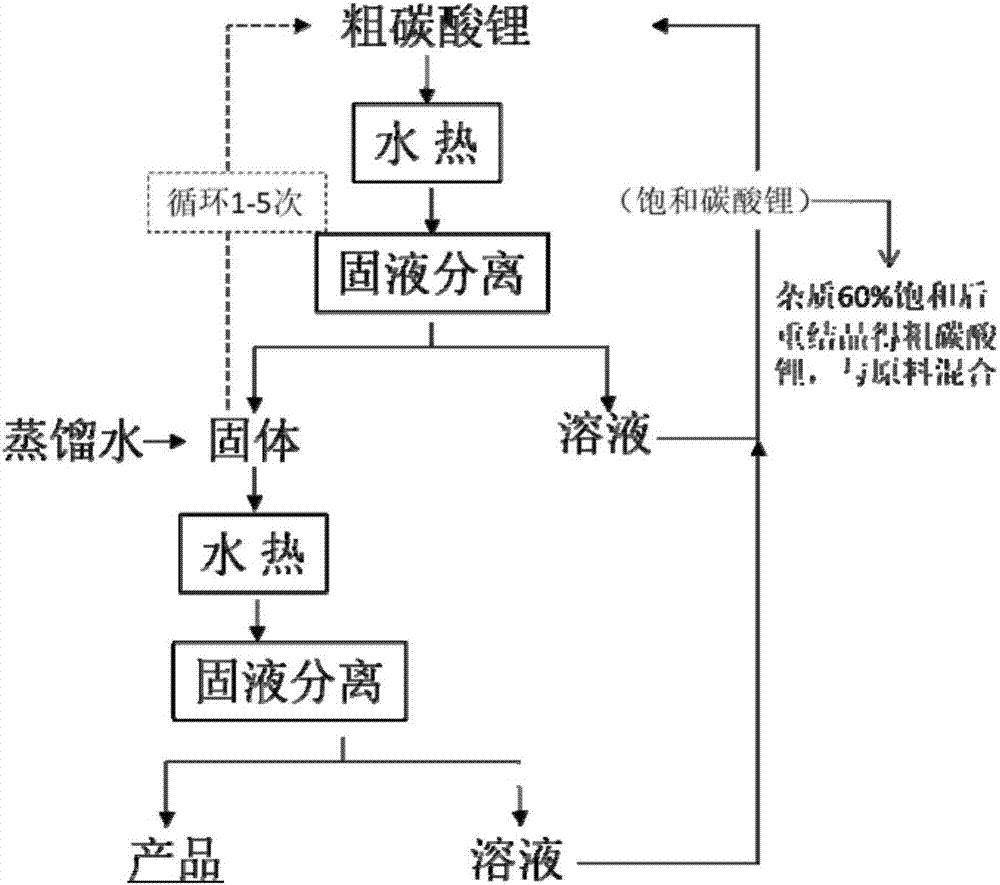
Hydrothermal purification method
InactiveCN107986302AReduce dissolution lossReduce churnBarium carbonatesLithium carbonates/bicarbonatesPurification methodsLithium carbonate
The invention provides a hydrothermal purification method. The method comprises hydrothermal purification, wherein hydrothermal purification comprises steps as follows: (1) a to-be-purified substanceand water are mixed, and slurry is obtained, wherein a target material in the to-be-purified substance is an insoluble substance and / or a slight soluble substance, and impurities in the to-be-purifiedsubstance are freely soluble substances; (2) the slurry is put in a hydrothermal reaction device, the hydrothermal reaction device is sealed to be heated to 110 DEG C or above for hydrothermal purification, and a hydrothermal purification product is obtained. The method also comprises the step (3): the hydrothermal purification product is subjected to solid-liquid separation, a solid and a liquidare obtained, and the solid is the purified to-be-purified substance. The method comprises simple procedures, the impurity removal rate and the yield of lithium carbonate are high, no new chemical reagents are used, no pollution is caused to the product, energy consumption is low, the production cost is low, and the method is applicable to industrial production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for harmless treatment of barium slag and synchronous recovery of barium carbonate
ActiveCN114380316AHelps maximize recyclingLow toxicityBarium carbonatesProcess efficiency improvementBarium saltSlag
The invention provides a method for harmlessly treating barium slag and synchronously recovering barium carbonate, and belongs to the technical field of solid waste treatment. The barium slag is barium-containing waste residues generated after dephosphorization is conducted on the high-phosphorus molten iron through a barium-based slag system, the main components of the barium slag are Ba3 (PO4) 2 and soluble barium salt, after the barium-containing waste residues generated after dephosphorization is conducted on the high-phosphorus molten iron are subjected to harmless treatment, barium carbonate precipitates are recycled, the toxicity of the barium-containing waste residues can be greatly reduced, and the production cost is reduced. According to the present invention, the process is simple, the environmental pollution is reduced, the processes are respectively optimized according to the different adding modes of the liquid-liquid reaction and the solid-liquid reaction during the treatment process, the pertinence is strong, the mixing uniformity of different reactants can be furthest improved, the maximum recovery of barium carbonate is easily achieved, and the advantages of energy saving and emission reduction are provided.
Owner:SOUTH CHINA INST OF ENVIRONMENTAL SCI MEP +1
Preparation method for monodisperse dumbbell-shaped barium carbonate particles
The invention discloses a preparation method for monodisperse dumbbell-shaped barium carbonate particles. The preparation method comprises the following steps: adding excessive carbonic ester into an alkaline barium ion solution with polyhydric alcohols to react; ending the reaction when the pH value of the solution is reduced to be 6.5-8; and filtering, and washing to obtain the monodisperse dumbbell-shaped barium carbonate particles. According to the preparation method, the carbonic ester is slowly hydrolyzed under an alkaline condition, and the released carbonate ions are bonded with the barium ions, so that the monodisperse dumbbell-shaped barium carbonate particles are assembled orderly under the control of the polyhydric alcohols. The reaction can be carried out at the room temperature, the preparation process is simple, the reaction raw materials are easily available, and the environment is friendly.
Owner:SOUTHWEST UNIVERSITY
Preparation method of ultrafine barium carbonate
ActiveCN108101095AAchieve preparationSimple processBarium carbonatesBulk chemical productionSlurryBall mill
The invention discloses a preparation method of ultrafine barium carbonate. The preparation method comprises the following steps: adding barites into ammonium carbonate slurry, and then putting into aball mill for wet milling to obtain barite slurry, putting the slurry in a rotary kiln to be calcined, then adding the calcined material into pure water, stirring and mixing, filtering to obtain first filtrate, adding the first filtrate into natural zeolite, filtering, introducing hydrogen sulfide and hydrogen fluoride solution into the obtained second filtrate, vacuum concentrating the obtainedthird filtrate, cooling, crystallizing to obtain barium hydroxide crystals, adding the barium hydroxide particles into a container with supercritical carbon dioxide, ultrasonically treating for 30 to50 min, then beginning to release the pressure, communicating the container and a dust collection apparatus, collecting solids, thus obtaining the ultrafine barium carbonate. The preparation method is simple in process, low in cost, fine in granularity of the obtained barium carbonate particles, less in waste water yield, high in purity and low in impurity content.
Owner:蒋央芳
Preparation method of nano barium carbonate slurry
ActiveCN108083313ASmall particle sizeImprove efficiencyBarium carbonatesNanotechnologySulfate radicalsReaction rate
The invention discloses a preparation method of nano barium carbonate slurry. The preparation method comprises the following steps: uniformly stirring 1.0 to 1.8 micrometer barium carbonate, deionizedwater and pentaerythritol or sodium polyacrylate, then pumping a mixture into a ceramic horizontal grinding machine by utilizing a metering pump, grinding by using zirconium oxide beads with a bead particle size of 100 to 1200 micrometers, controlling a flow rate of the metering pump at 3L / min and a rotation speed of the grinding machine at 1450 r / min, thus obtaining the nano-scale barium carbonate slurry. By adopting the nano barium carbonate slurry obtained by the invention, the reaction rate of the barium carbonate and sulfate radicals when the nano barium sulfate is prepared can be apparently increased, the sulfate radicals in the sodium chloride saline water can be more effectively removed, the application efficiency of the barium carbonate in removing impurities of the saline watercan be effectively increased, the obtained nano barium carbonate is used as a raw material to prepare nano precipitate barium sulfate, the obtained nano precipitate barium sulfate powder is smaller inparticle size, and no soluble barium ions and chlorine ions can be detected in the nano precipitate barium sulfate powder.
Owner:云浮鸿志新材料有限公司
Large-particle titanium silicalite molecular sieve with excellent diffusion performance and preparation method thereof
ActiveCN111470517AImprove diffusion abilityImprove diffusivityBarium carbonatesMaterial nanotechnologyMolecular sieveTitanium
The invention provides a large-particle titanium silicalite molecular sieve TS-1 with excellent diffusion performance. The morphology of the molecular sieve is a cuboid, and the longest side size is 4-20 [mu]m; and a large number of spherical holes with the diameter of 70-100 nm are formed inside. The invention also provides a preparation method of the molecular sieve. Carbonate is used as a hardtemplate of a hole structure, tetrapropylammonium bromide is used as a microporous template agent, and the molecular sieve is prepared by an acid treatment method. The hole structure of the molecularsieve prepared by the method is more beneficial to diffusion of macromolecules, so that the limitation of intrinsic micropore channels of the TS-1 molecular sieve on catalytic reaction is overcome, and non-framework titanium can be removed and the removal of the framework titanium is inhibited through acid treatment. Therefore, compared with the conventional microporous TS-1, the titanium silicalite molecular sieve provided by the invention has higher catalytic activity on a larger molecular selective oxidation reaction and has better stability on a smaller molecular selective oxidation reaction.
Owner:DALIAN UNIV OF TECH +1
Preparation method of barium carbonate with large specific surface area
The invention discloses a preparation method of barium carbonate with a large specific surface area, and relates to the technical field of preparation of barium carbonate. The preparation method comprises the steps of at normal temperature, dissolving barium chloride in deionized water to prepare a solution, and cooling the barium chloride solution to 1-4 DEG C after filtration; at normal temperature, dissolving sodium carbonate in deionized water to prepare a solution, and cooling the sodium carbonate solution to 1-4 DEG C after filtration; and adding the sodium carbonate solution of 2 DEG C into a reaction kettle, placing the reaction kettle into ultrasonic synthesis equipment, adding the barium chloride solution into the reaction kettle at a constant speed while performing stirring in the reaction kettle at 2-5 DEG C, pouring materials obtained after completion of the reaction into a washing barrel, pouring out the supernatant after natural clarification, adding deionized water, performing washing for seven times, then performing solid-liquid separation by using a centrifuge, putting a solid barium carbonate wet material obtained after separation into an electric heating constant-temperature air blast drying oven for drying, and performing screening through a sieve, thereby obtaining the barium carbonate with a large specific surface area. The preparation method is low in energy consumption and is easy to operate, and other pollutants are not introduced into the preparation process.
Owner:广州建丰稀土有限公司
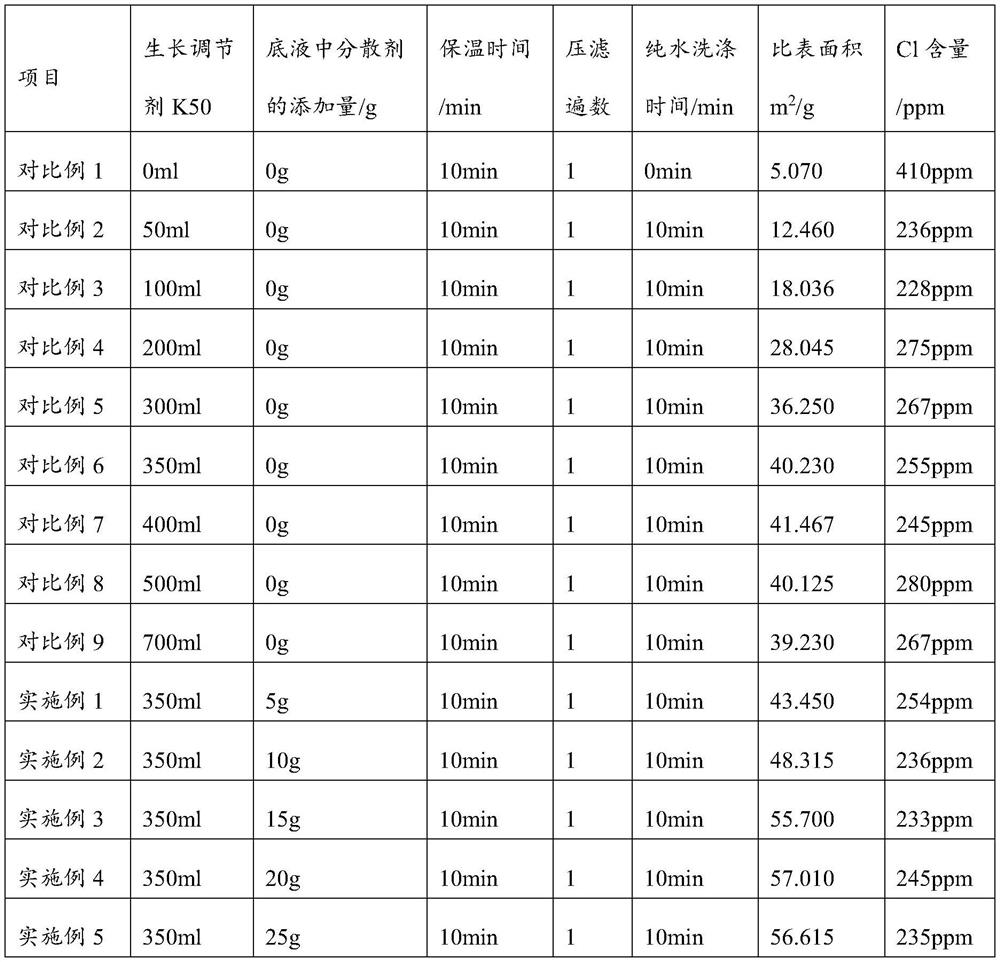
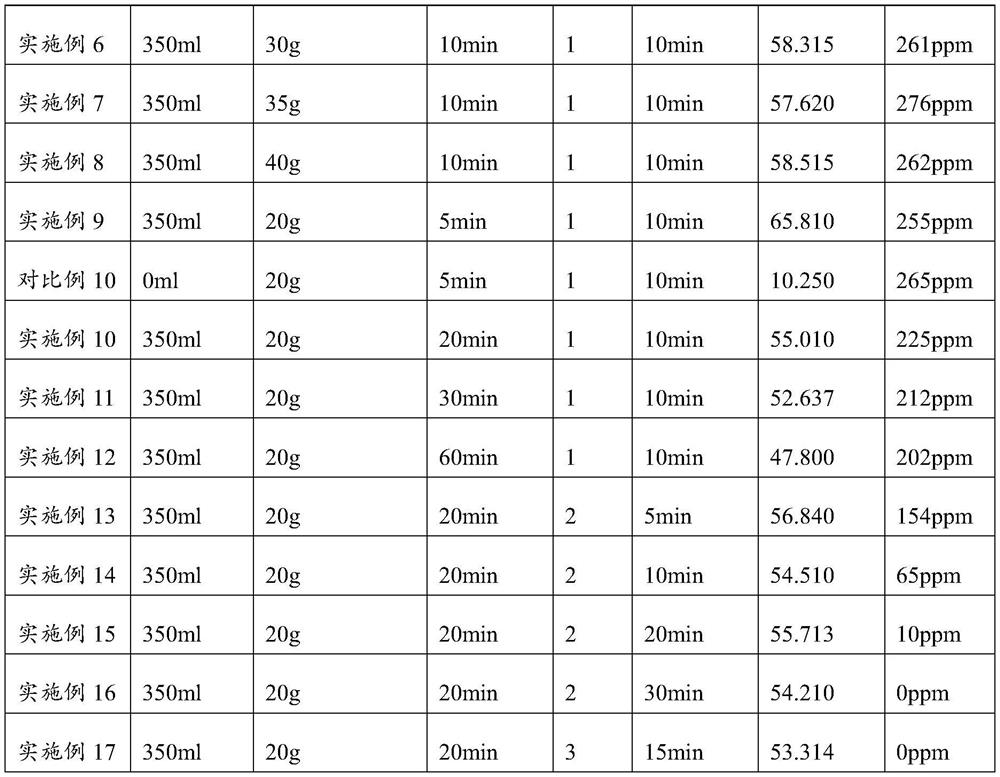
Large-specific-surface-area barium carbonate as well as preparation method and application thereof
PendingCN114516652ASmall particle sizeAvoid reunionBarium carbonatesFixed capacitor dielectricBarium dichlorideBarium salt
The invention provides large-specific-surface-area barium carbonate as well as a preparation method and application thereof, and relates to the technical field of materials. The preparation method of the large-specific-surface-area barium carbonate provided by the invention comprises the following steps: adding a barium salt solution and a carbonate solution into a dispersing agent solution for reaction, and carrying out heat preservation treatment to prepare the large-specific-surface-area barium carbonate. Wherein a specific growth regulator is dissolved in the barium salt solution, so that the agglomeration phenomenon can be effectively inhibited; the barium salt solution is added into the dispersing agent solution in one step compared with the carbonate solution, so that the Ostwald ripening phenomenon and the re-agglomeration phenomenon of generated barium carbonate are avoided; barium chloride which is cheaper than barium hydroxide is used as a raw material, so that the raw material cost is greatly saved. The preparation method is simple in operation process and mild in reaction condition, industrial production can be achieved, the specific surface area of the prepared barium carbonate can reach 50 m < 2 > / g or above, and the requirement of the market for large-specific-surface-area barium carbonate can be met.
Owner:SHANDONG SINOCERA FUNCTIONAL MATERIAL CO LTD
A kind of preparation method of large specific surface area barium carbonate
The invention discloses a preparation method of barium carbonate with a large specific surface area, and relates to the technical field of preparation of barium carbonate. The preparation method comprises the steps of at normal temperature, dissolving barium chloride in deionized water to prepare a solution, and cooling the barium chloride solution to 1-4 DEG C after filtration; at normal temperature, dissolving sodium carbonate in deionized water to prepare a solution, and cooling the sodium carbonate solution to 1-4 DEG C after filtration; and adding the sodium carbonate solution of 2 DEG C into a reaction kettle, placing the reaction kettle into ultrasonic synthesis equipment, adding the barium chloride solution into the reaction kettle at a constant speed while performing stirring in the reaction kettle at 2-5 DEG C, pouring materials obtained after completion of the reaction into a washing barrel, pouring out the supernatant after natural clarification, adding deionized water, performing washing for seven times, then performing solid-liquid separation by using a centrifuge, putting a solid barium carbonate wet material obtained after separation into an electric heating constant-temperature air blast drying oven for drying, and performing screening through a sieve, thereby obtaining the barium carbonate with a large specific surface area. The preparation method is low in energy consumption and is easy to operate, and other pollutants are not introduced into the preparation process.
Owner:广州建丰稀土有限公司
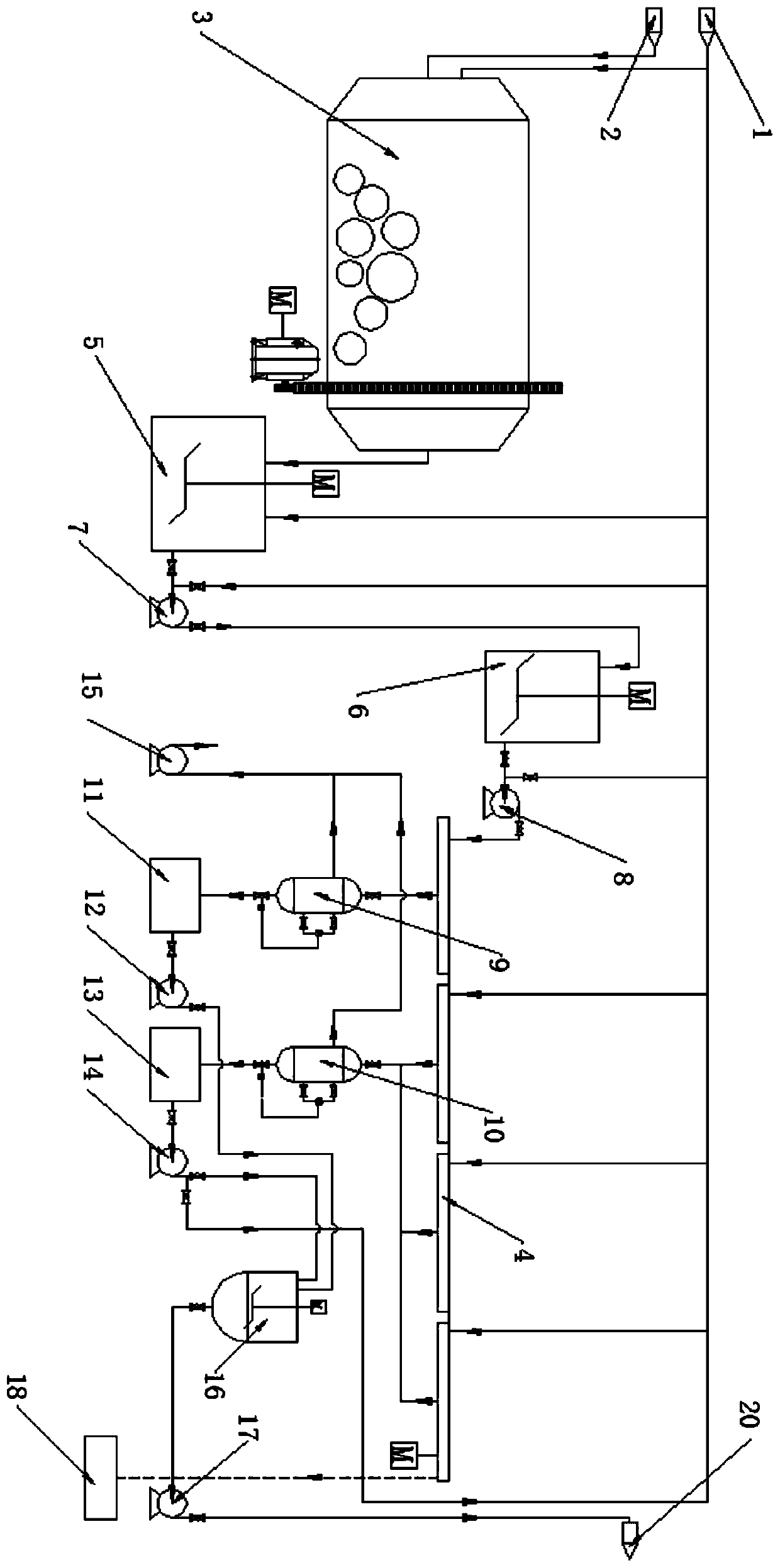
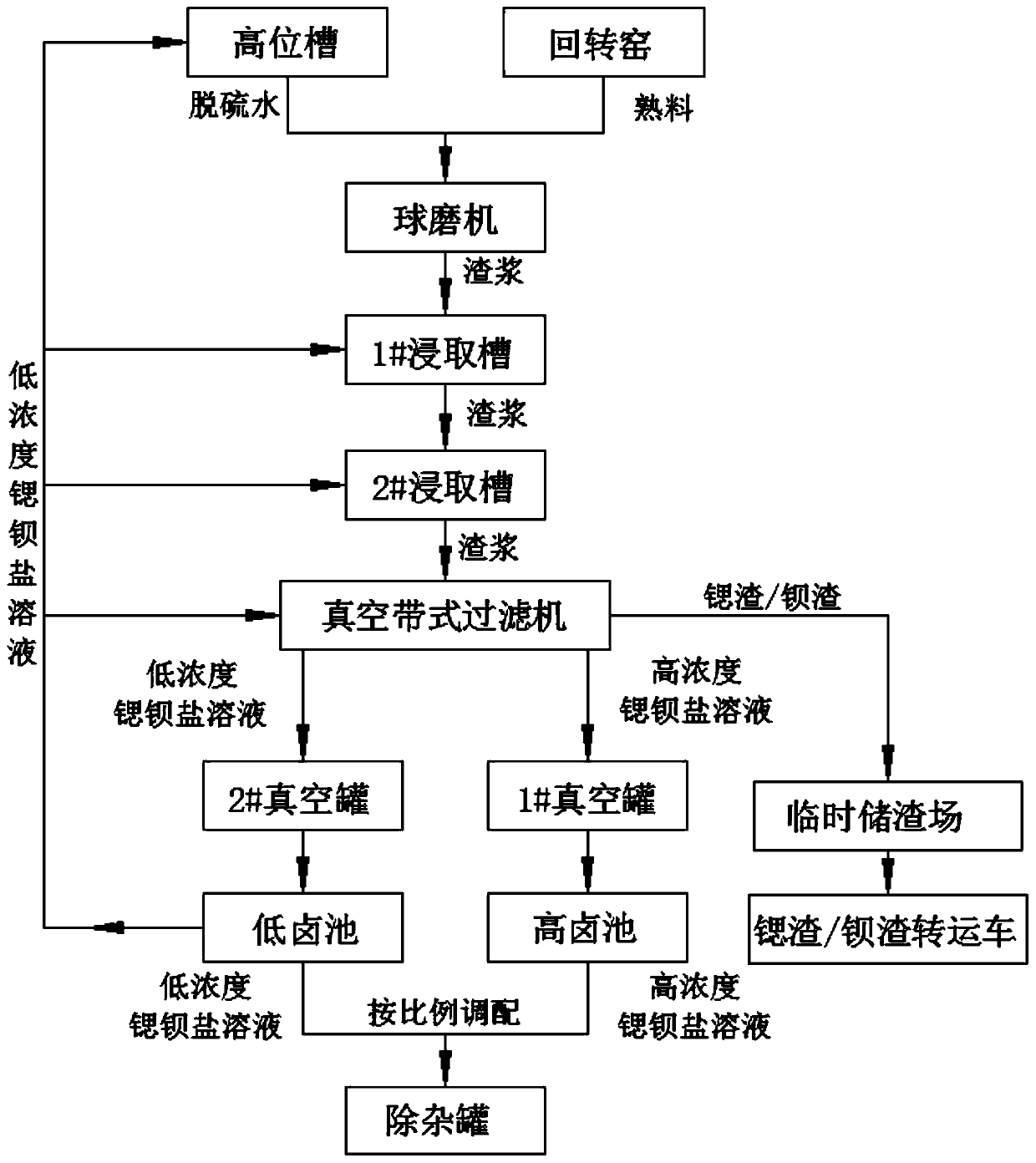
Strontium-barium salt continuous leaching and deslagging production system and production process
InactiveCN110510582ASolve temperature problemsSolve the problem of dustBarium carbonatesStrontium carbonatesHigh concentrationEnvironmental resistance
The invention discloses a strontium-barium salt continuous leaching and deslagging production system, which comprises a ball mill, leaching tanks, a vacuum belt type pumping filter and the like and realizes continuous leaching and deslagging of strontium-barium salt. The invention also discloses a strontium-barium salt continuous leaching and deslagging production process, specifically, a roastedmaterial is added into a recovered strontium-barium salt solution, then grinding is carried out in a ball mill, and a 1# leaching tank and a 2# leaching tank are employed to leach the ground materialstwice, thus solving the severe factors of high temperature, much dust, much water mist and the like at a leaching station; the leached materials are filtered on the vacuum belt type pumping filter interms of a front section and a back section, and a high-concentration strontium-barium salt solution and a low-concentration strontium-barium salt solution with stable concentration can be obtained respectively, and the brine of low-halogen pool is added at the latter half section of the vacuum belt type pumping filter for washing, thus improving the filtering effect, effectively recovering strontium and barium elements, reducing waste, improving the post safety and environmental protection, and eliminating the safety and environmental protection hidden danger of an overall plant area.
Owner:CHONGQING KINGLONG FINE STRONTIUM CHEM
Preparation method of barium carbonate and barium carbonate prepared by using same
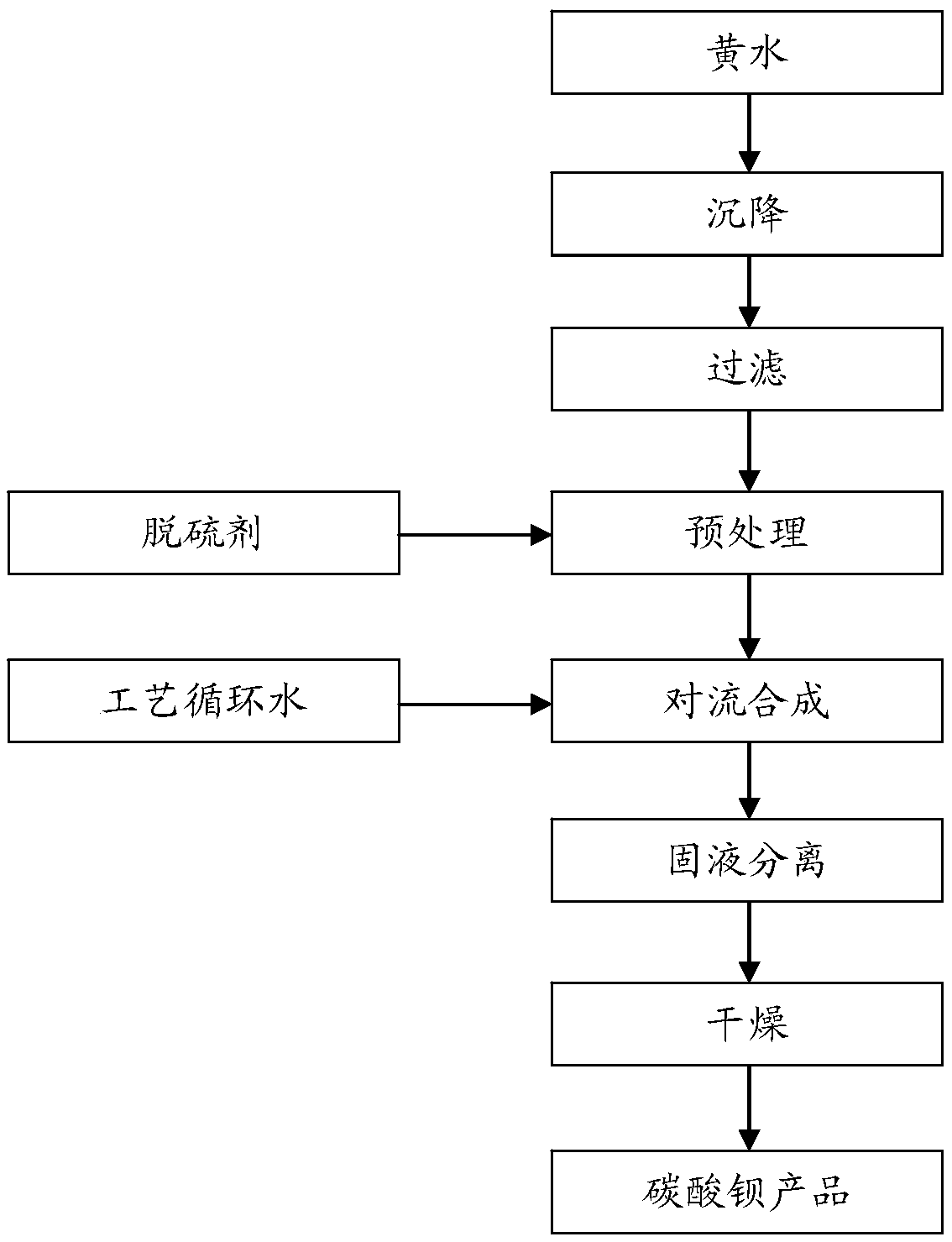
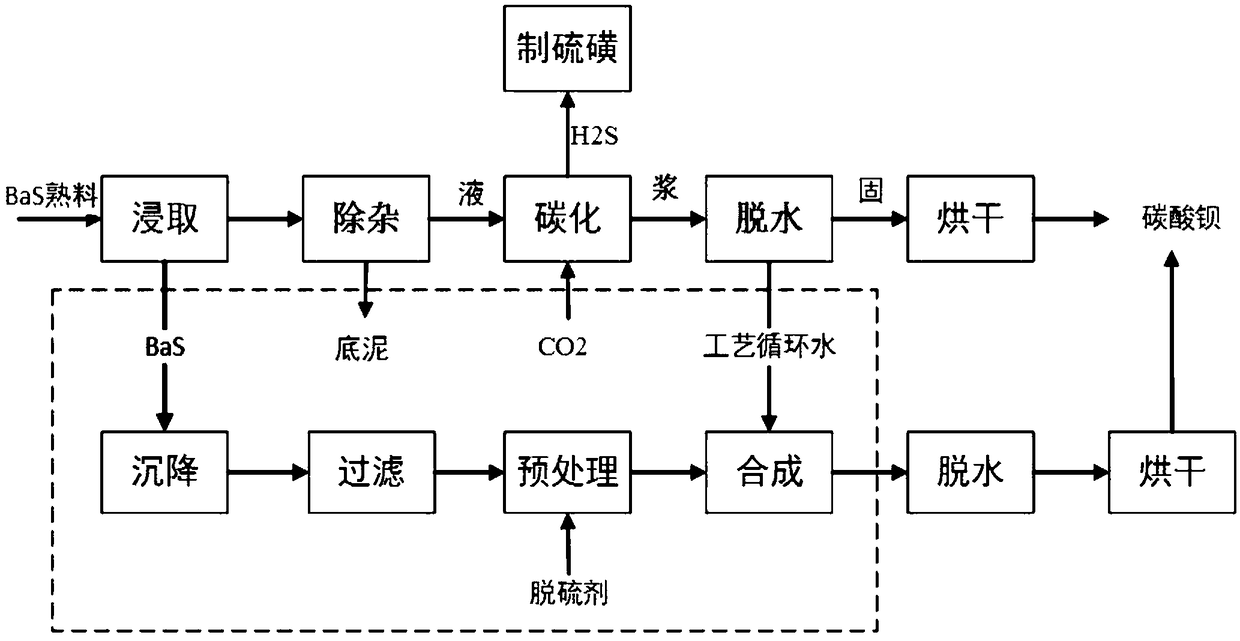
The invention provides a preparation method of barium carbonate. The preparation method comprises the following steps: naturally settling yellow water, and filter-pressing, to obtain the pure yellow water; adding a desulfurizing agent into the pure yellow water and pre-processing, wherein the desulfurizing agent is sodium hydroxide solution or soluble sodium salt solution, while a Na<+> concentration in reaction solution reaches 0.01 mol / L to 0.025 mol / L, stopping pre-processing, to obtain the pre-processed yellow water; enabling the pre-processed yellow water to perform a continuous convection current synthetic reaction with technology circulating water, while a pH value of reaction slurry reaches 9.5-11.5, stopping the reaction, performing continuous sedimentation curing on the reactionslurry, and performing solid-liquid separation, collecting a solid; washing and drying the collected solid, to obtain a barium carbonate product. The invention further provides the barium carbonate product prepared by using the method. The content of the barium carbonate is greater than or equal to 99.48wt%. The method is capable of, through performing decarbonization recovery on the technology circulating water, preparing the industrial barium carbonate, improving a recovery rate of the barium carbonate production to the greatest extent, reducing the generation of solid wastes, and in complywith the requirements of national resource conservation and emission reduction.
Owner:GUIZHOU REDSTAR DEVING
Method for improving the recovery of cesium-131 from barium carbonate
The present invention provides a method for improving the recovery of cesium-131 (Cs-131) from barium (Ba) carbonate. Uses of the Cs-131 purified by the method include cancer research and treatment, such as for the use in brachytherapy. Cesium-131 is particularly useful in the treatment of faster growing tumors.
Owner:ISORAY MEDICAL INC
Method for preparing barium carbonate and the product obtained by the method
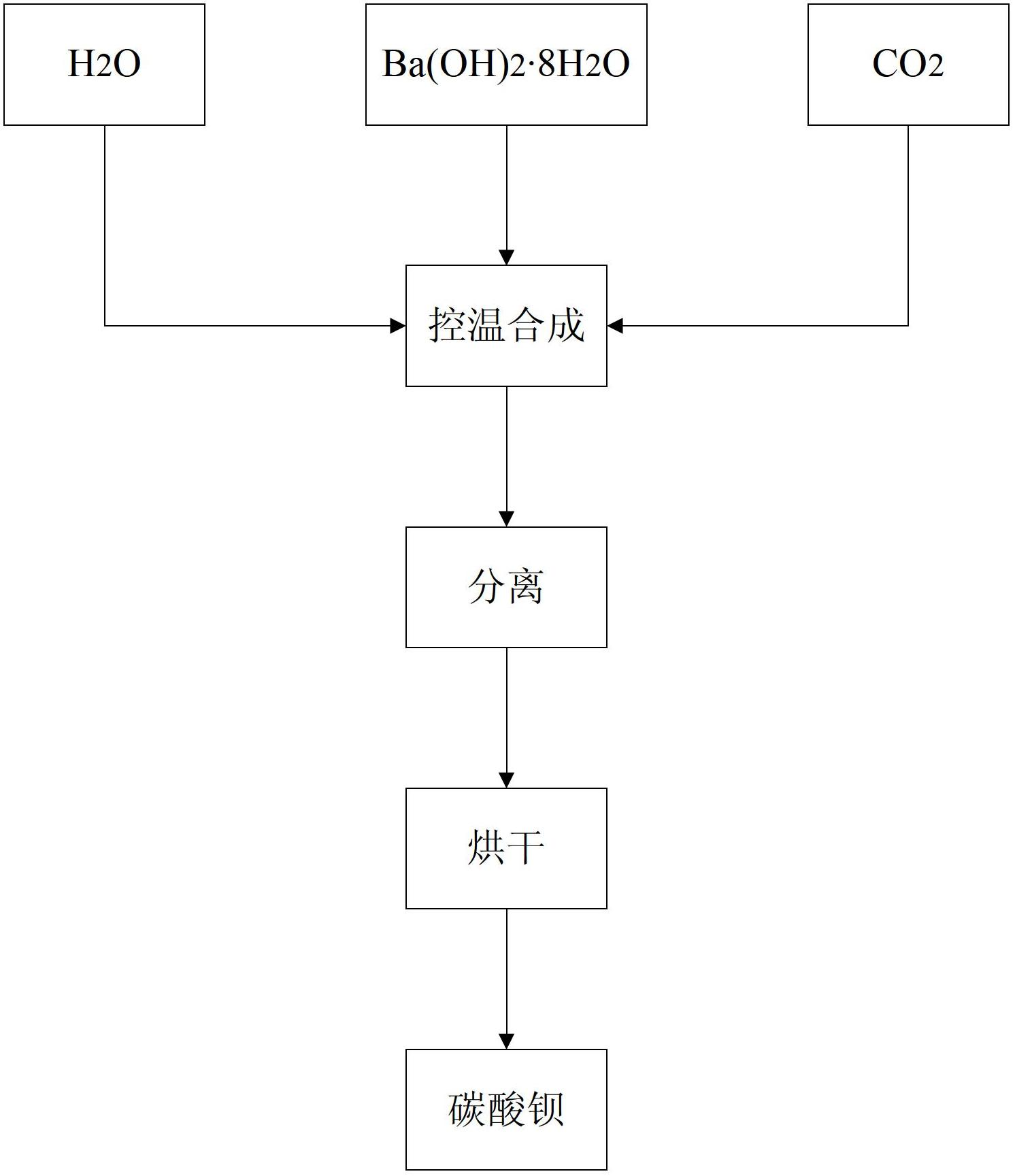
InactiveUS20130316175A1High purityFunction increaseBarium carbonatesMagnesium carbonatesPhysical chemistryBarium hydroxide octahydrate
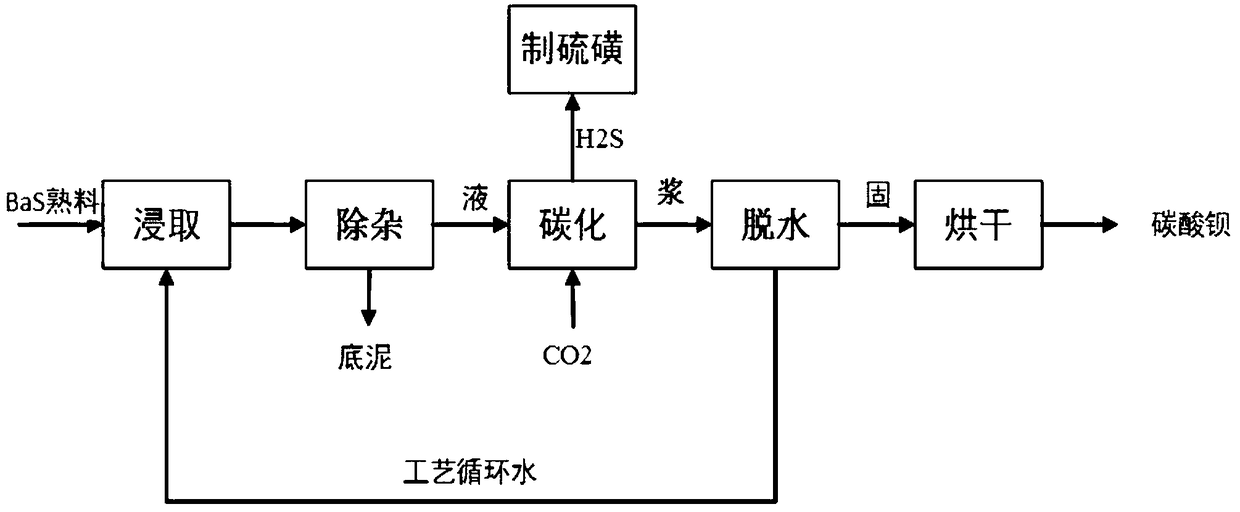
The present invention relates to a method for preparing barium carbonate and the barium carbonate product obtained by the method. The method for preparing barium carbonate comprises: (1) Adding barium hydroxide octahydrate crystal into the ice-water bath and mixing them, and controlling the temperature to be within 0˜5° C.; (2) Introducing carbon dioxide into the mixture obtained from the step (1), till pH value of the reaction solution reaches 6.0-7.0; (3) Carrying out solid-liquid separation for the solid-liquid mixture obtained from the step (2), drying the obtained solid so as to obtain barium carbonate product. The present invention method solves the problem in the prior art that the character of low strontium and the character of large specific surface area can not co-exist, which makes the property of barium carbonate product can not affect the sintering temperature, and thus the property material is improved.
Owner:GUIZHOU REDSTAR DEVING +1
Generally spherical barium carbonate particles, and method for producing generally spherical barium carbonate particles
ActiveCN102369160AImprove uniformityPigmenting treatmentBarium carbonatesParticulatesSpherical shaped
Owner:SAKAI CHEM IND CO LTD
Production process of barium carbonate
PendingCN112624172ACreate pollutionSimple production processBarium carbonatesBarium solutionGas liquid reaction
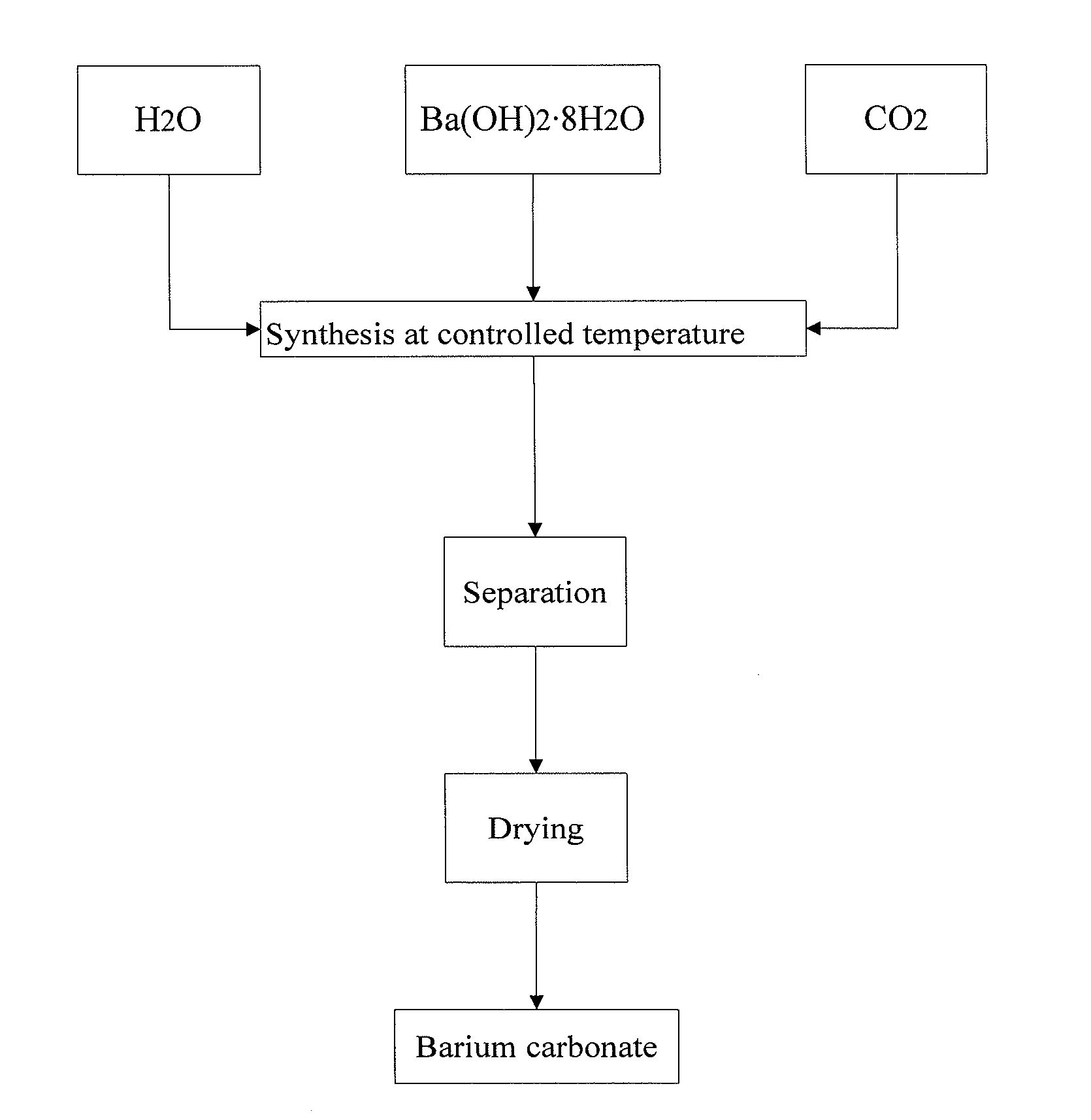
The invention relates to a production process of barium carbonate. The production process comprises the following steps: adding barium hydroxide into high-purity water, and dissolving in a dissolving pump to obtain a barium hydroxide solution; adding liquid ammonia into the barium hydroxide solution, and stirring for 10-60 minutes at a constant temperature of 30-80 DEG C; introducing carbon dioxide into the barium hydroxide solution added with the liquid ammonia through a micro reaction device in a stirring state, and stopping introducing the carbon dioxide when the pH value is equal to 6-9, so as to obtain a barium carbonate solution; heating the prepared barium carbonate solution to enable the barium carbonate solution to fully react; precipitating and filtering to obtain a barium carbonate precipitate; and drying the barium carbonate precipitate in a dryer to obtain high-purity electronic-grade barium carbonate. According to the invention, carbon dioxide and barium hydroxide are used as raw materials for gas-liquid reaction, the production process is simple, no by-product is produced in the reaction, the product purity is high, and no pollution is caused to the environment.
Owner:宜昌华昊新材料科技有限公司
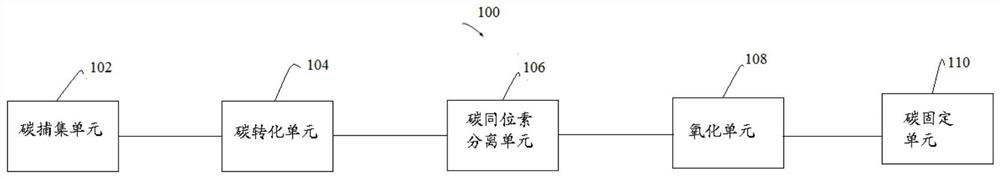
Method for recovering 14C in 14C-containing exhaust gas
The present disclosure relates to a method for recovering 14C in a 14C-containing exhaust gas, the method The comprises: capturing CO2 in the 14C-containing exhaust gas to obtain a CO2-rich gas; converting CO2 in the CO2-rich gas into CO and / or CH4 to obtain a CO-rich and / or CH4-rich gas; performing isotope separating on the CO-rich and / or CH4-rich gas to obtain a < 14 > CO-rich and / or < 14 > CH4-rich gas; oxidizing < 14 > CO and / or < 14 > CH4 in the < 14 > CO-rich and / or < 14 > CH4-rich gas into < 14 > CO2 to obtain < 14 > CO2-rich gas; and fixing the < 14 > CO2 in the < 14 > CO2-rich gas. According to the invention, efficient recovery and reutilization of 14C in radioactive waste gas can be realized.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
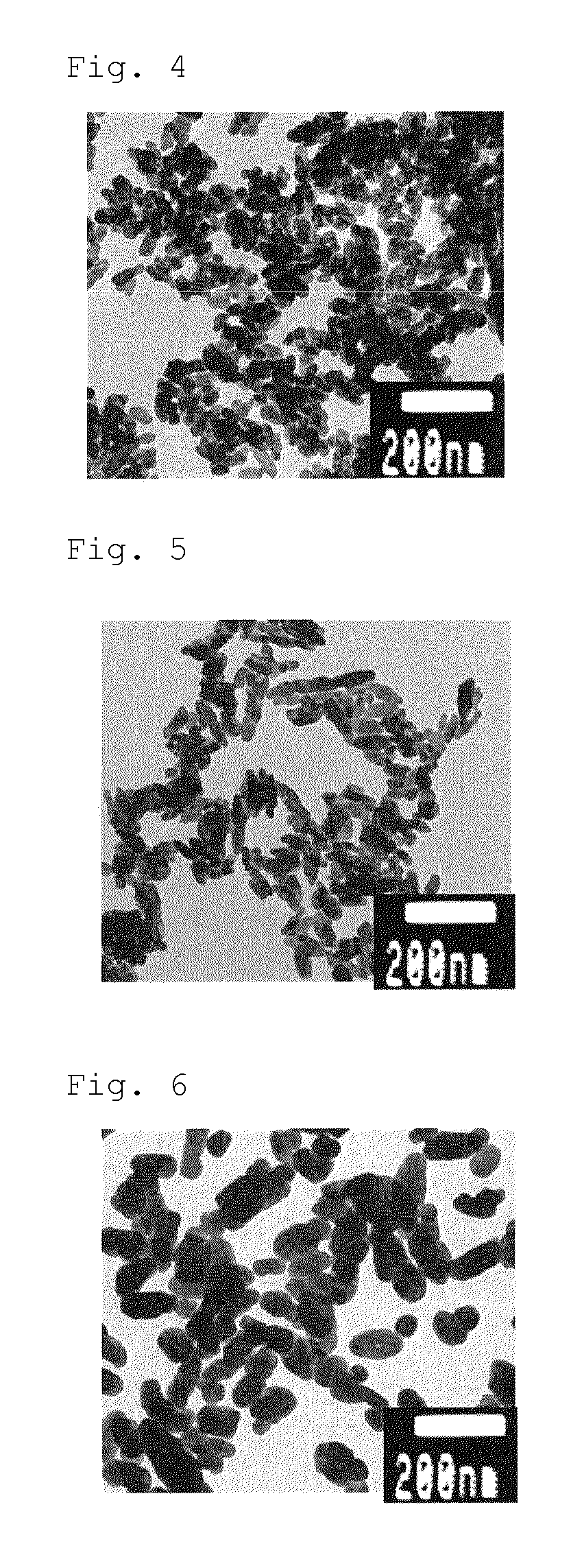
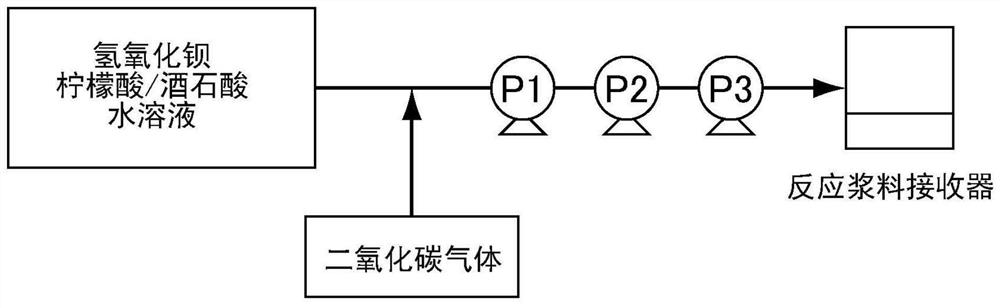
Barium carbonate production method
The present invention provides a method whereby barium carbonate that is so fine as to exceed a specific surface area of 45 m2 / g can be produced economically and with little burden on the environment.The present invention relates to a barium carbonate production method which is characterized by comprising: a step of adding (a) citric acid and / or a salt thereof to at least one barium compound selected from the group consisting of barium hydroxide, barium chloride, barium nitrate, barium acetate, and barium oxide; a step of reacting (b) carbon dioxide and / or a carbonate compound therewith; anda step of adding (c) at least one among a polybasic carboxylic acid represented by formula (1), an anhydride thereof, or a salt thereof, wherein the percentage by mole A added of (a), the citric and / or the salt thereof, and the percentage by mole B added of (c), the at least one among the polybasic carboxylic acid represented by formula (1), the anhydride thereof, or the salt thereof, with respectto 100% by mole of the barium atoms in the barium compound satisfies the following formulas (I) and (II). HOOC-X-(COOH)n...(1) (In the formula, X represents a divalent or a trivalent linking group; the linking group represents a saturated hydrocarbon group having one or two carbons that may have a hydroxyl group as a substituent, an unsaturated hydrocarbon group having two carbons, or a group derived from a benzene ring; and n represents 1 or 2.) 6.0 <= A+B <= 16...(I) 0.01 <= A / B <= 7.0... (II).
Owner:SAKAI CHEM IND CO LTD
Method for recovering barium in wastewater containing barium isobutyric acid through carbonate precipitation method
InactiveCN108394923AReduce COD valueBarium carbonatesWater contaminantsHigh concentrationChemical oxygen demand
The invention discloses a method for recovering barium in wastewater containing barium isobutyric acid through a carbonate precipitation method. The method comprises the following steps of (1) dropwise adding a carbonate saturated solution into the barium isobutyric acid wastewater, and thus producing a barium carbonate sediment, wherein the molar ratio of barium ions to carbonate in the barium isobutyric acid wastewater is 1:(1 to 2); during a dropwise adding process, controlling the temperature to be less than or equal to 30 DEG C; (2) filtering a reaction liquid obtained through the step (1) to obtain filter liquid and a filter cake, wherein the filter cake is barium carbonate; collecting. The method provided by the invention can be used for treating the high-concentration barium isobutyric acid wastewater; after treatment through the method, the barium in the wastewater can be completely recovered, isobutyric acid radical roots are transformed into valuable isobutyric acid, and thewastewater is low in COD (Chemical Oxygen Demand) value and near colorless.
Owner:宁波永顺精细化工有限公司
Normal-temperature synthesis method of electronic-grade high-purity barium carbonate
The invention discloses a normal-temperature synthesis method of electronic-grade high-purity barium carbonate.The method comprises the steps of barium carbonate dissolution and purification, barium carbonate synthesis and barium carbonate washing and purification.A purified barium carbonate solution is dropwise added into a food-grade ammonium bicarbonate solution under the normal temperature to synthesize barium carbonate, and the washing frequency of the barium carbonate is reduced to three.The synthesis temperature of the high-purity barium carbonate is reduced from the traditional 60 DEG C to normal temperature; on the basis of ensuring that the synthesized barium carbonate meets the electronic-grade high-purity requirement, energy is saved, emission is reduced, and the cost is reduced.
Owner:BAOJI QINLONG ELECTRONICS SCI & TECH CO LTD
Preparation method of high-purity barium carbonate
ActiveCN110002485AImprove conversion rateFully contactedBarium carbonatesSulfur preparation/purificationAcid washingNitrogen gas
The invention discloses a preparation method of high-purity barium carbonate. The preparation method comprises the following steps: (1) crushing barite into powder, carrying out alkali washing, acid washing and water washing, and finally carrying out drying to obtain barite powder; (2) adding the barite powder into a reactor, introducing nitrogen to replace air in the reactor, continuously introducing nitrogen, carrying out heating to 1000-2000 DEG C, then introducing a mixed gas of sulfur vapor and nitrogen into the reactor, carrying out reacting for a period of time, and carrying out coolingto room temperature to obtain crude barium sulfide; (3) dissolving the crude barium sulfide in deionized water, and then carrying out filtering to obtain a barium sulfide clear solution; and (4) under the condition of 0-100 DEG C and a stirring rotating speed of 0-1000 r / min, introducing CO2 gas into the barium sulfide clear solution or slowly adding a sodium carbonate solution to carry out a precipitation reaction to obtain the high-purity barium carbonate. The preparation method disclosed by the invention is high in raw material conversion rate and meets the green production idea, and purity of the prepared barium carbonate is higher than 99.5%.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Superfine nano barium carbonate and preparation method thereof
PendingCN113816414ASmall particle sizeHigh purityBarium carbonatesNanotechnologyAlcoholMixed materials
The invention relates to the technical field of functional ceramic raw materials, in particular to superfine nano barium carbonate and a preparation method thereof. The preparation method of the superfine nano barium carbonate comprises the following steps of A) mixing an alcoholic solution and barium hydroxide octahydrate to obtain an alcoholic solution of barium hydroxide octahydrate, B) stirring the alcoholic solution of barium hydroxide octahydrate and dimethyl carbonate to react, stopping stirring when the pH value is less than or equal to 8, adding ammonia water, and standing to obtain a mixed solution, and C) carrying out solid-liquid separation on the mixed material liquid, and drying to obtain the superfine nano barium carbonate. The mixed solution of alcohol and water is used as a reaction medium, a barium source and dimethyl carbonate are subjected to one-step reaction at normal temperature and normal pressure to synthesize the sphere-like superfine nano barium carbonate powder, the particle size of the powder is small, and the purity of the powder is high; meanwhile, secondary high-temperature heat treatment can be avoided, steps are simplified, and energy is saved.
Owner:福建贝思科电子材料股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com