Barium carbonate production method
A manufacturing method and technology of barium carbonate are applied in directions such as barium carbonate, calcium carbonate/strontium/barium, etc. to achieve the effect of less load on the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
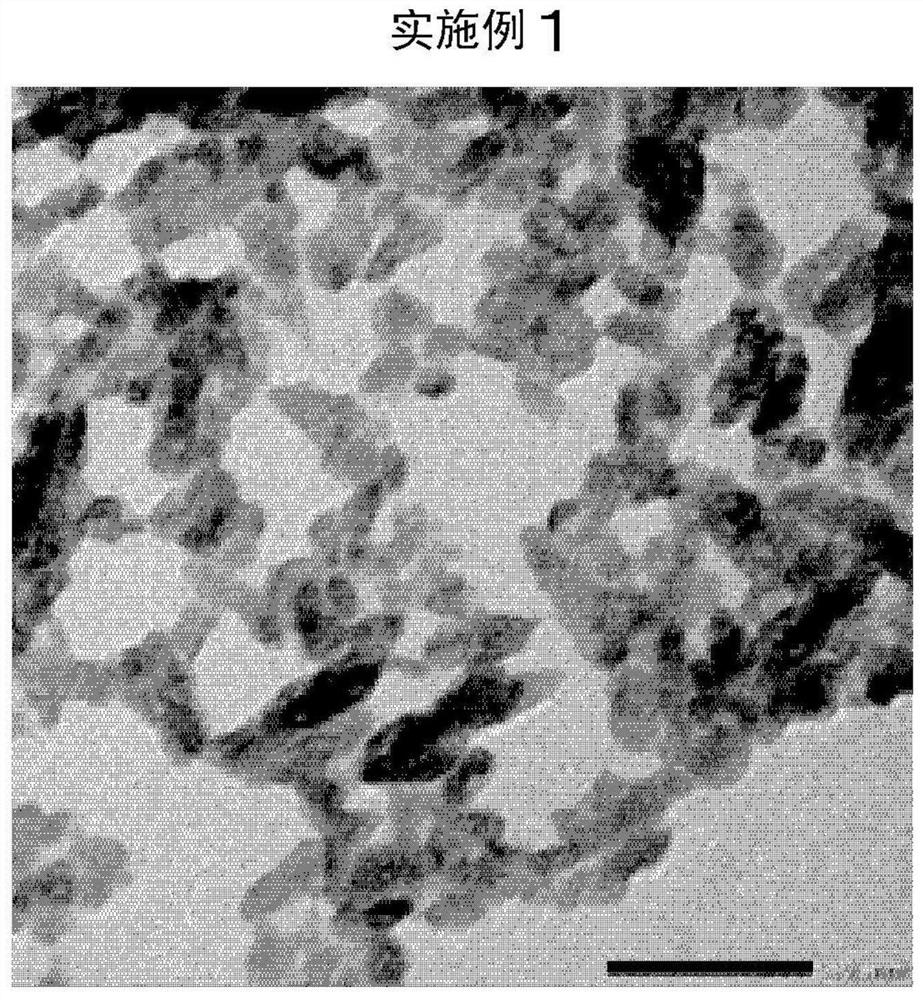
Embodiment 1
[0121] After dissolving barium hydroxide octahydrate in pure water, 2.5 mol% citric acid monohydrate salt solution (manufactured by Wako Pure Chemical Industries, Ltd.) and 5 mol% tartaric acid were added to the barium ions in barium hydroxide. Aqueous solution (manufactured by Wako Pure Chemical Industries, Ltd.).
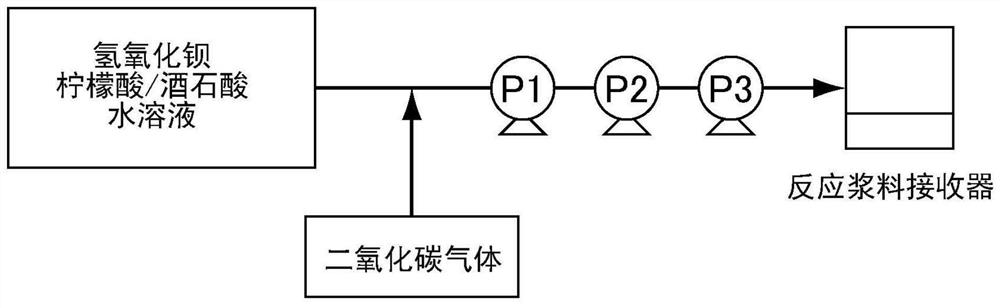
[0122] Next, it was diluted with pure water so that the final concentration of barium hydroxide octahydrate was 72.5 g / L to prepare a barium hydroxide / citric acid / tartaric acid aqueous solution (raw material A). The liquid temperature at this time was adjusted to 32°C. figure 1 In the shown reaction apparatus, the raw material A was charged into the suction port of the pump P1 at a flow rate of 400 ml / min. Simultaneously, the raw material A was injected into the flow path of the pump P1 so that the pH became 6.0 to 7.0, and carbon dioxide gas was blown in at 4.2 L / min to implement the reaction. Such as figure 1 As shown in the reaction apparatus, three connec...
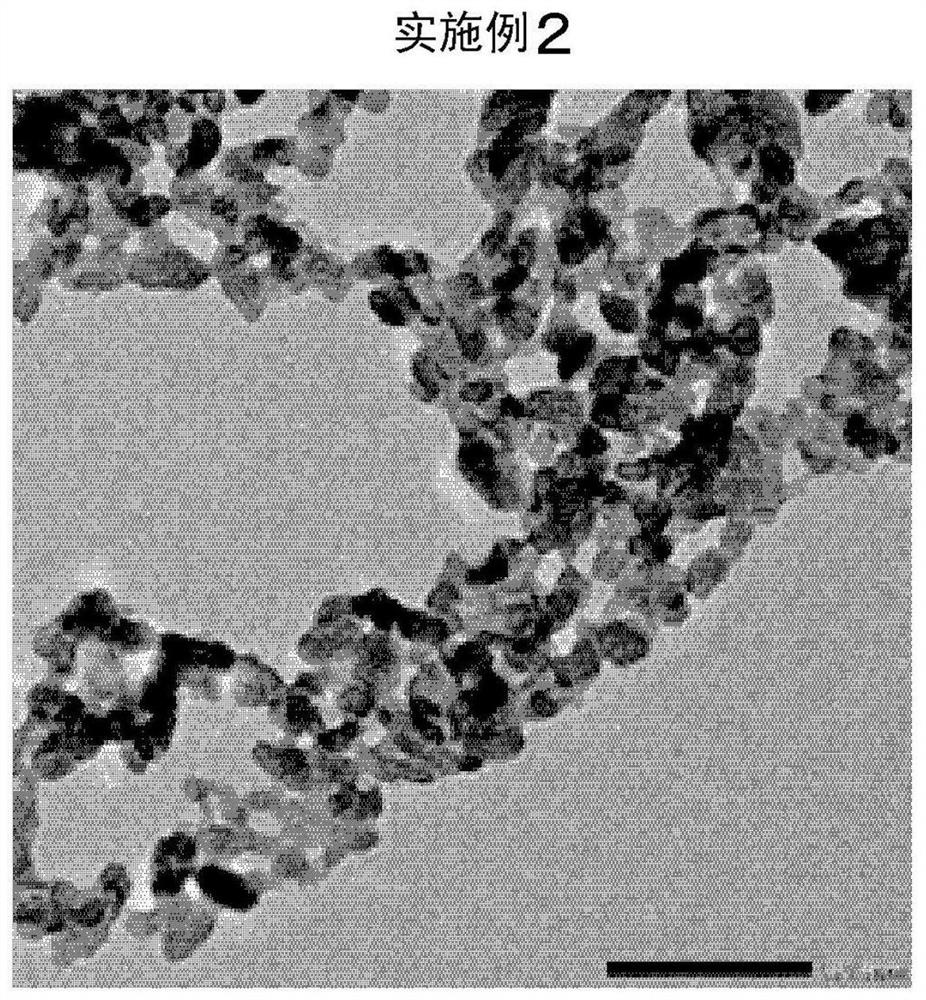
Embodiment 2
[0125] Except having changed 5.0 mol% of tartaric acid in Example 1 into 7.5 mol%, it carried out similarly to Example 1. The results are shown in Table 1. In addition, electron micrographs are shown in image 3 .
Embodiment 3
[0127] In Example 1, it carried out similarly to Example 1 except having changed 2.5 mol% of citric acid into 5.0 mol%, and 5.0 mol% of tartaric acid into 2.5 mol%. The results are shown in Table 1. In addition, electron micrographs are shown in Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com