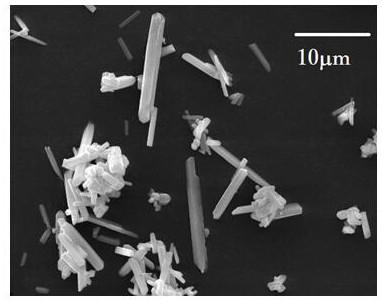
Preparation method of high-purity barium carbonate
A high-purity barium carbonate and sodium carbonate technology, which is applied in the preparation/purification of barium carbonate and sulfur, calcium carbonate/strontium/barium, etc., can solve the problem that by-products are not resourced and utilized, are environmentally friendly, consume a lot, and chloride ions are not easy to remove and other issues to achieve maximum value utilization and increase conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
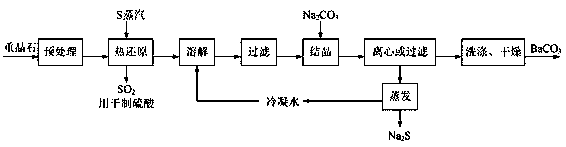
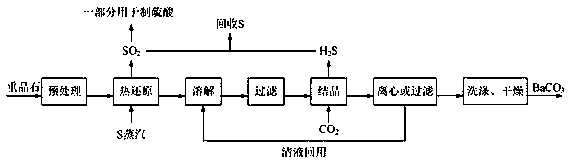
[0028] A preparation method of high-purity barium carbonate, specifically comprising the following steps:
[0029] (1) Pretreatment: crush the barite into a powder with a particle size of less than 50 μm, then wash with alkali, pickle and water, and finally dry to obtain barite powder;
[0030] In this step, alkali washing can effectively remove the alkali-soluble impurities in the barite, acid washing can effectively remove the acid-soluble impurities in the barite, washing with water can effectively remove the barite soluble impurities, and the barite in the barite Barium sulfate is neither soluble in alkali nor acid. It can be washed with alkali first, then pickled, and finally washed with water, or it can be acid washed first, then washed with alkali, and finally washed with water.
[0031] (2) Thermal reduction reaction: Add the barite powder obtained in step (1) into the thermal reduction reactor, and after introducing nitrogen gas to replace the air in the reactor, con...
Embodiment 1
[0053] The barite crushed to a particle size of less than 50 μm is washed sequentially with sodium hydroxide solution, hydrochloric acid solution and deionized water, and then dried at an environment higher than 90 ° C for 24 hours; take 30 g of dried barite powder Put it in the reactor, pass in nitrogen to replace the air in the reactor, continue to pass in nitrogen, raise the temperature to 1400°C, and then pass in the mixed gas of sulfur vapor and nitrogen, the volume ratio of sulfur vapor and nitrogen is 1:4, and the mixture is controlled The gas flow rate is 0.3-0.5L / min, the space time of the mixed gas in the reactor is 4.8-8min, and the crude barium sulfide is produced after 1.5h of reaction; then the crude barium sulfide is dissolved in 1L deionized water, and the barium sulfide is produced after filtering Supernatant liquid, the molar concentration of barium sulfide in the barium sulfide clear liquid obtained by detection and analysis is 0.11mol / L; Get 300ml of barium ...
Embodiment 2
[0055] The barite crushed to a particle size of less than 50 μm is washed sequentially with sodium hydroxide solution, hydrochloric acid solution and deionized water, and then dried at an environment higher than 90°C for 24 hours; take 50g of dried barite powder Put it in the reactor, pass in nitrogen to replace the air in the reactor, continue to pass in nitrogen, raise the temperature to 1200°C, and then pass in the mixed gas of sulfur vapor and nitrogen, the volume ratio of sulfur vapor and nitrogen is 1:5, and the mixture is controlled The gas flow rate is 0.25 ~ 0.4L / min, the space time of the mixed gas in the reactor is 6 ~ 9.6min, and the crude barium sulfide is produced after 1 hour of reaction, then the crude barium sulfide is dissolved in 1L deionized water, and the barium sulfide is produced after filtration Supernatant liquid, the molar concentration of barium sulfide in the barium sulfide clear liquid obtained by detection and analysis is 0.18mol / L; Get 200ml of ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com