Preparation method of ultrafine barium carbonate
A technology of barium carbonate and ammonium carbonate slurry, which is applied in the field of powder materials, can solve the problems of high impurity content and large particle size of barium carbonate, and achieve the effect of low impurity content, fine particle size and avoiding agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
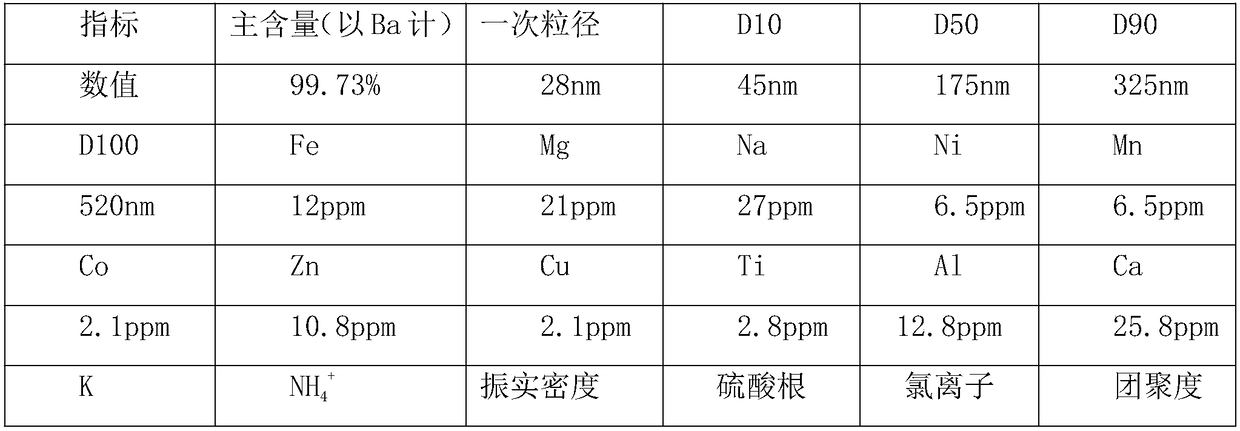
Embodiment 1
[0037] A kind of preparation method of superfine barium carbonate, it is the following steps:
[0038] (1) Add barite to ammonium carbonate slurry, then put it into a ball mill for wet grinding to obtain barite slurry, then put the slurry into a rotary kiln for calcination at a temperature of 385°C, and use an induced draft fan at the same time The air in the rotary kiln is taken away, the pressure in the rotary kiln is maintained at 0.92 atmospheres, the calcination time is 3.5 hours, then the calcined material is added to pure water and stirred for 1.8 hours, and then filtered to obtain the first filtrate and the first Filter residue, add the first filtrate to natural zeolite, mix and stir for 1.8 hours at a temperature of 55°C, then filter to obtain the second filtrate and second filter residue, pass the second filtrate into hydrogen sulfide, and react at a temperature of 43°C for 0.75 hours , then add hydrogen fluoride solution, react at a temperature of 93° C. for 2.5 hou...
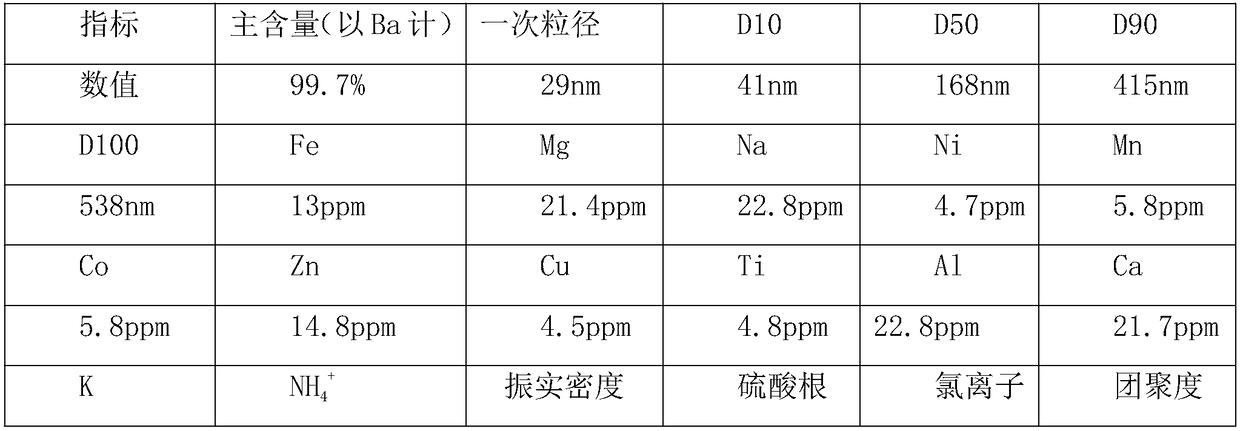
Embodiment 2
[0050] A kind of preparation method of superfine barium carbonate, it is the following steps:
[0051] (1) Add barite to ammonium carbonate slurry, then put it into a ball mill for wet grinding to obtain barite slurry, then put the slurry into a rotary kiln for calcination at a temperature of 385°C, and use an induced draft fan at the same time The air in the rotary kiln is taken away, the pressure in the rotary kiln is maintained at 0.92 atmospheres, the calcination time is 3.7 hours, then the calcined material is added to pure water and stirred for 1.2 hours, and then filtered to obtain the first filtrate and the first Filter residue, add the first filtrate to natural zeolite, mix and stir for 1.8 hours at a temperature of 52°C, then filter to obtain the second filtrate and second filter residue, pass the second filtrate into hydrogen sulfide, and react at a temperature of 42°C for 0.75 hours , then add hydrogen fluoride solution, react at a temperature of 92° C. for 2.5 hou...
Embodiment 3
[0062] A kind of preparation method of superfine barium carbonate, it is the following steps:
[0063](1) Add barite to ammonium carbonate slurry, then put it into a ball mill for wet grinding to obtain barite slurry, then put the slurry into a rotary kiln for calcination at a temperature of 385°C, and use an induced draft fan at the same time The air in the rotary kiln is taken away, the pressure in the rotary kiln is maintained at 0.92 atmospheres, the calcination time is 3.7 hours, then the calcined material is added to pure water and stirred for 1.2 hours, and then filtered to obtain the first filtrate and the first Filter residue, add the first filtrate to natural zeolite, mix and stir for 1.8 hours at a temperature of 52°C, then filter to obtain the second filtrate and second filter residue, pass the second filtrate into hydrogen sulfide, and react at a temperature of 42°C for 0.75 hours , then add hydrogen fluoride solution, react at a temperature of 92° C. for 2.5 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com