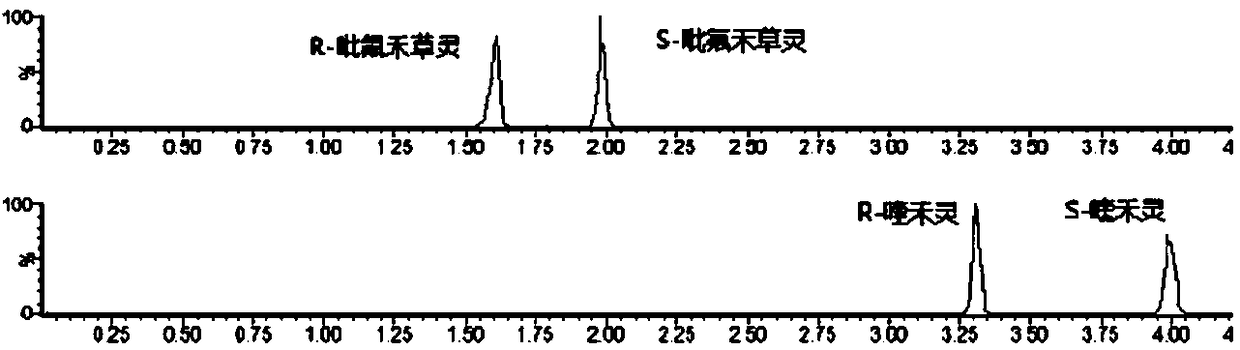
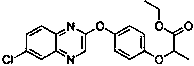
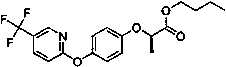
Method for splitting and determining chiral pesticide quizalofop-ethyl and fluazifop-butyl enantiomers by super high performance combined chromatography-tandem mass spectrometry
A technology of fluazifop-pyr and ultra-high-efficiency phase combination, which is applied in the field of analytical chemistry and can solve problems such as poor separation of fluazifop-pyr and long analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] 1. Instruments and reagents:
[0037] Acetonitrile, ethanol, and methanol are chromatographic grade reagents, and sodium citrate and sodium chloride are analytical reagents; distilled water meets the requirements of first-grade water in GB / T 6682.
[0038] Waters TQD quadrupole tandem mass spectrometer; water bath constant temperature oscillator; Swiss Mettler AE 163 electronic balance (sensitivity: 0.0001g).
[0039] 2. Sample handling:
[0040] Accurately weigh 2 g of the ground tobacco powder sample into a 50 mL capped centrifuge tube, add 10 mL of water, add 10 mL of acetonitrile after soaking, then place the centrifuge tube on a vortex mixer and vibrate at a rate of 2000 rpm 5 min. Then add 5 g of anhydrous magnesium sulfate, 1 g of sodium chloride, 1 g of sodium citrate and 0.5 g of disodium hydrogen citrate to the centrifuge tube, and immediately oscillate at a rate of 2000 rpm for 5 min, then centrifuge at 6000 rpm for 3 min; pipette 1.0 mL of the supernata...
example 2
[0049] Grain samples were selected as described in Example 1, and fluazifop-p-p and quizalofop-p-p were not detected in the samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com