Borondifluoride complexes of curcominoid compounds, method of preparation and uses thereof
A compound, boron difluoride technology, applied in the field of boron difluoride complexes, to achieve the effects of enhanced optical properties, high optical brightness, and enhanced fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] Embodiment 1: the synthesis of compound 4
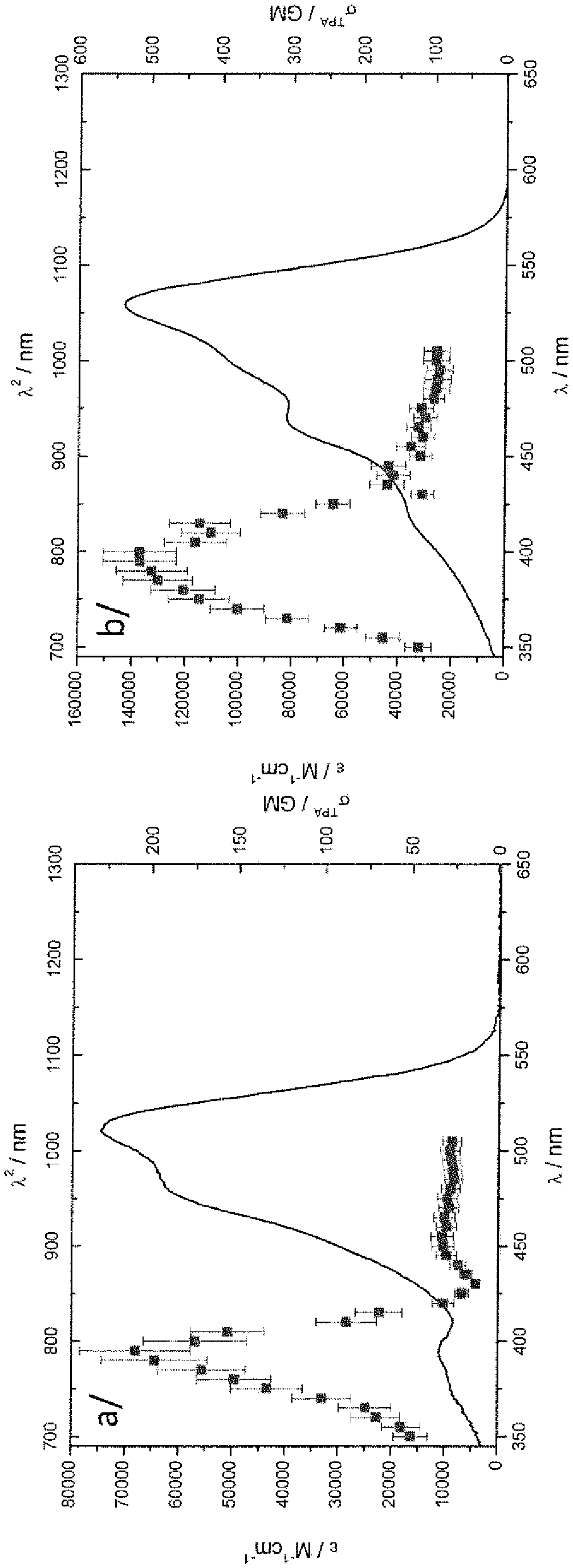
[0237] The synthesis of compound 4 is described below. The two-photon excited fluorescence (TPEE) properties of 4 were characterized in solution. The preparation and fluorescence emission of organic nanoparticles obtained using 4 are also described. This allows to give an example of compounds as defined in the present invention having improved emission both in solution and in the solid state.
[0238]
[0239] The synthesis of compound 4 first requires the preparation of compound A. This intermediate was prepared by the Noevenger reaction using an excess of acetylacetone (acetylacetone / aldehyde 3:1) to provide compound A in a reasonable yield of 60% (G. Mann, L. Beyer and A. Arrieta, Z. Chem., 1987, 27, 172-173). Then, the reaction of two equivalents of A with 1,3,5-tris(n-octoxy)benzene afforded 4 in 34% yield.
[0240] Complexation with boron difluoride was carried out by reacting compound B with a slight excess of...
Embodiment 2
[0250] Example 2: Electrochemical, photophysical and solid-state optical properties of compound 4 and comparison with other compounds
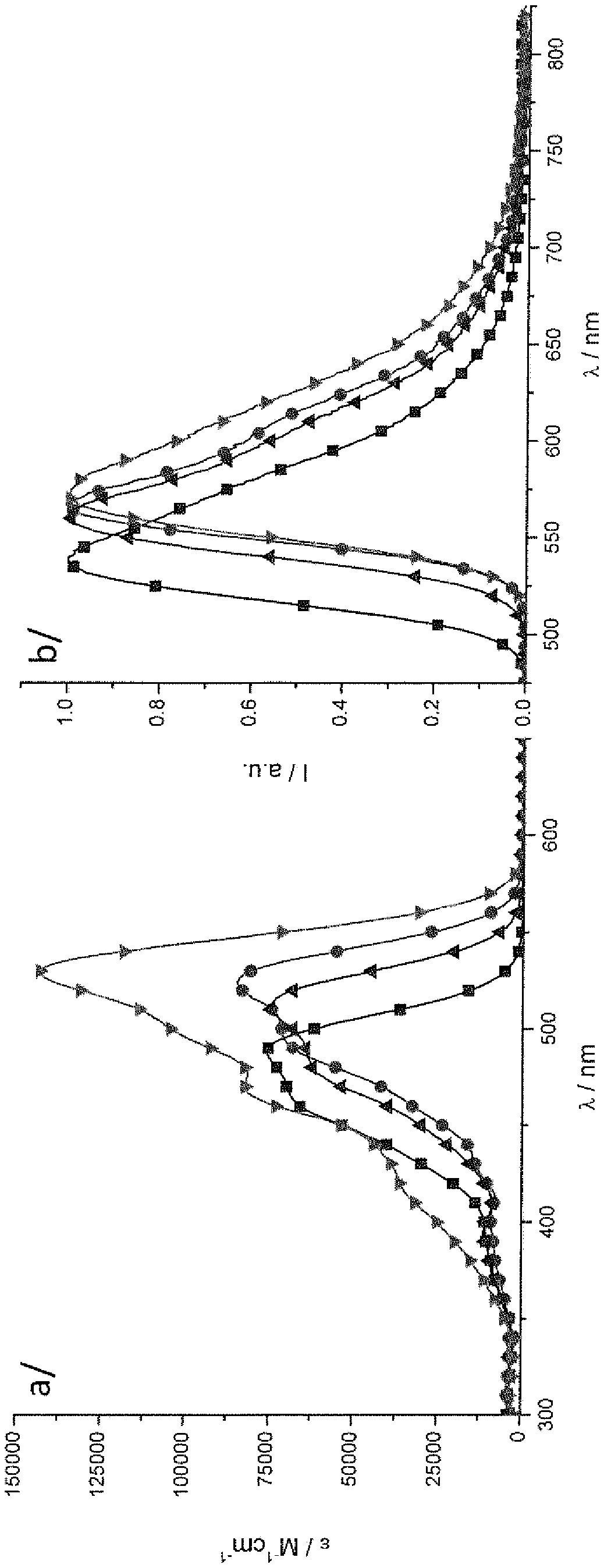
[0251] Here the electrochemical and optical properties of four compounds in organic solvents are reported: borondifluoride complexes of three monocurcuminoids (named X, Y, and Z) and borondifluoride complexes of the biscurcuminoid system Compound complex compound 4 compared. The two-photon excited fluorescence properties (TPEF) of four compounds in solution were characterized.
[0252] The molecular structures of borodifluoride curcumin derivatives X, Y, and Z are shown below.
[0253]
[0254] 2.1 Comparison of electrochemical properties
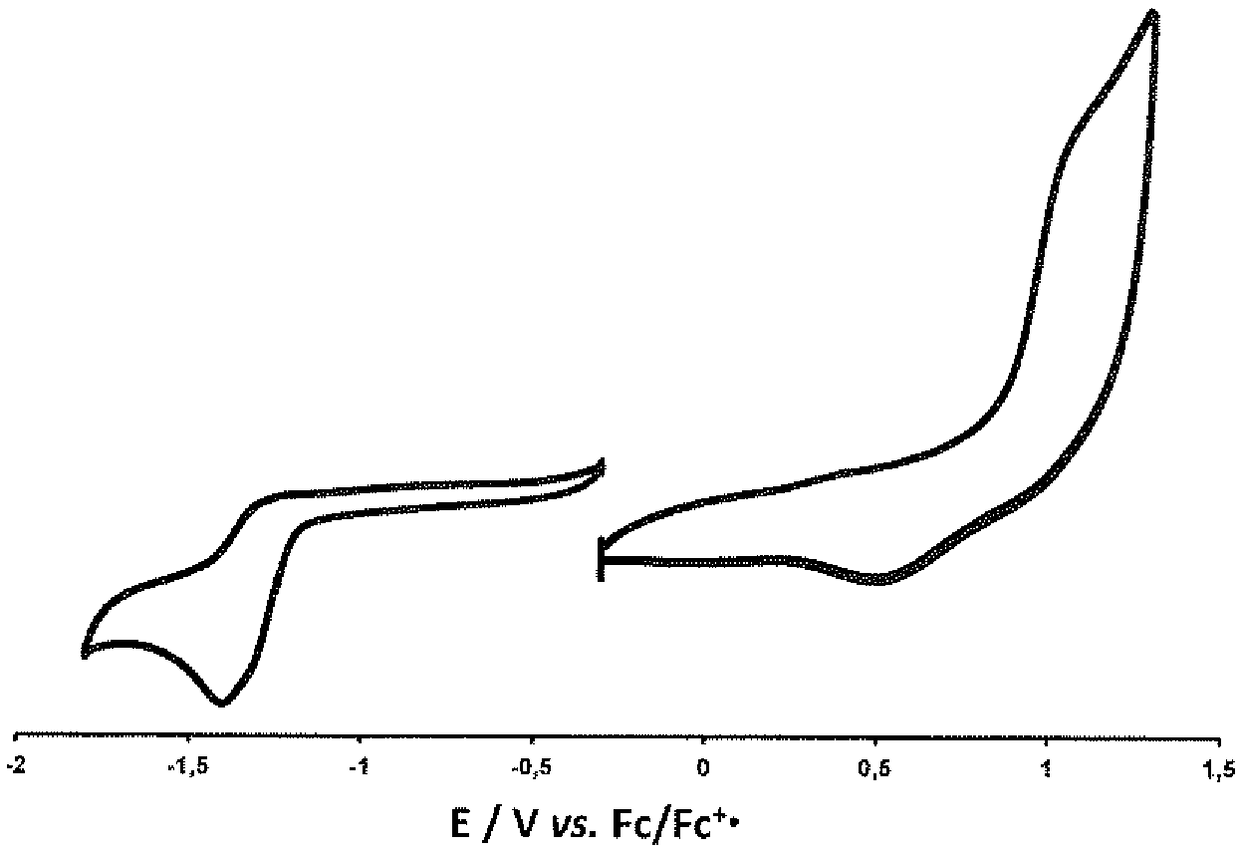
[0255] Electrochemical properties of compounds X, Y, Z and 4 in the presence of 0.1M (n-Bu) 4 NPF 6 studied in dichloromethane (DCM) solution.
[0256] figure 1 The cyclic voltammetry curve (CV) is given in, and Table 1 lists the oxidation and reduction half-wave potential values (E 1 / 2 , r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com