A kind of dihalogenated coumarin-platinum (ii) complex and its synthesis method and application
A technology of coumarin substitution and synthesis method, which is applied in the direction of platinum-based organic compounds, platinum-group organic compounds, and compounds containing elements of group 8/9/10/18 of the periodic table, etc., which can solve the toxic and side effects of platinum-based drugs, Poor water solubility, non-specific targeting, etc., to achieve superior in vitro anti-tumor activity and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
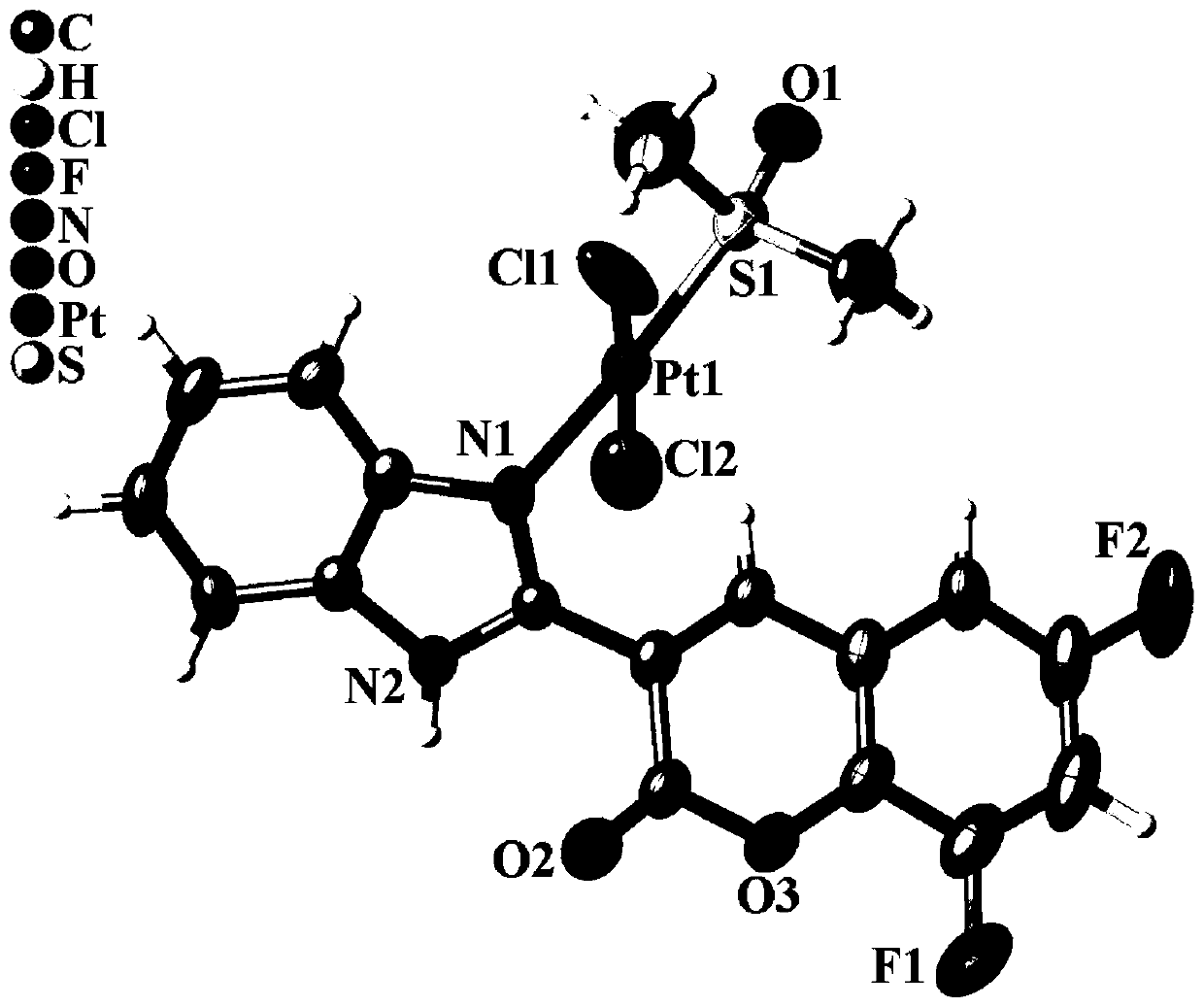
[0037] Accurately weigh 1.0 mmol of dichloro-bis(dimethylsulfoxide) platinum (II) and 1.0 mmol of ligand FFBC, dissolve dichloro-bis(dimethyl sulfoxide) platinum (II) Dissolve the ligand FFBC in 3 mL of methanol in 1 mL of dimethyl sulfoxide solution, then mix the two solutions and put them into a polytetrafluoroethylene reactor, react at 100 ° C for 48 hours, cool to room temperature, and precipitate The yellow granular solid was washed with distilled water, methanol and ether in turn, and the compound 1 was obtained after vacuum drying with a yield of 80.3%.
[0038] The resulting yellow granular crystals were identified:
[0039] (1) Infrared spectrum, its spectrogram is as follows Figure 4 Shown:
[0040] IR(KBr):3242,3039,1727,1590,1431,1378,1309,1241,1139,1095,969,737,677,442cm -1 .
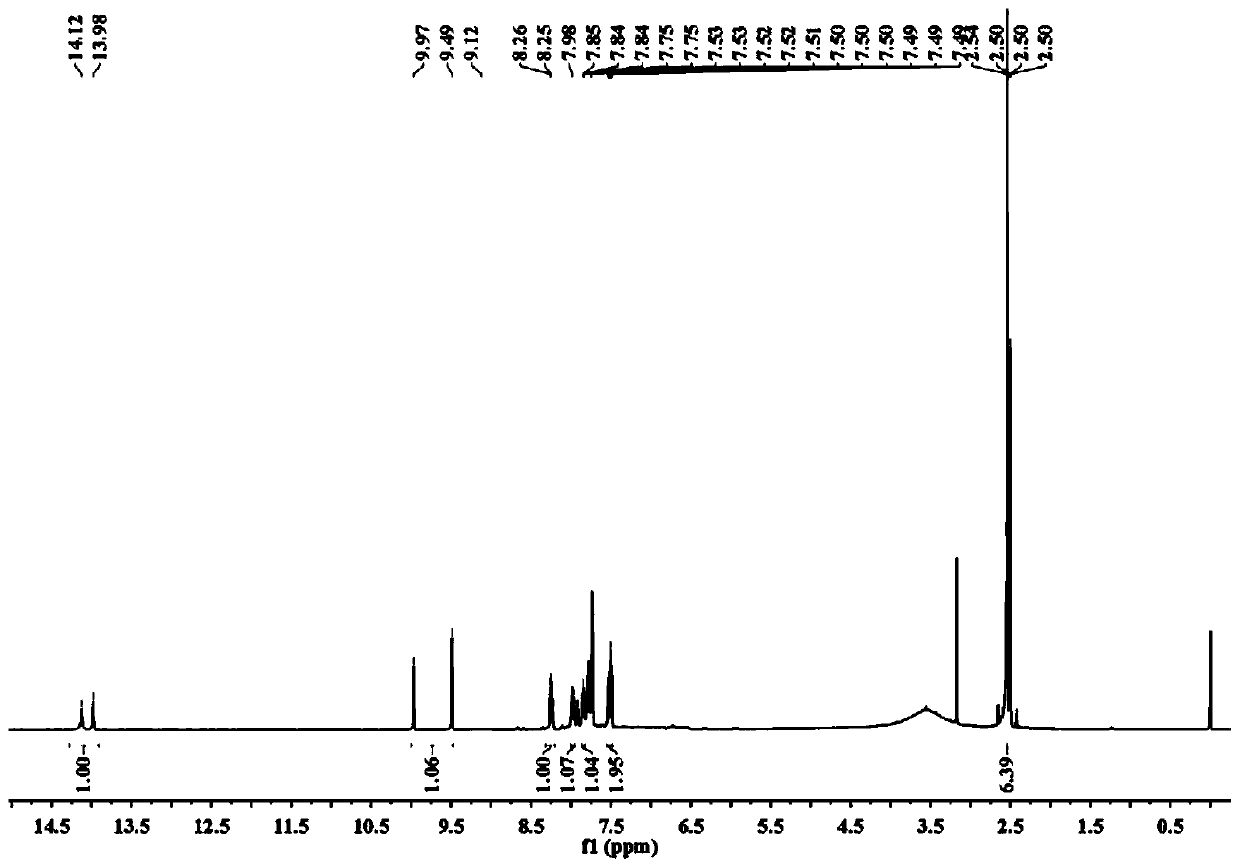
[0041] (2) Proton NMR spectrogram, its spectrogram is as figure 1 shown.
[0042] 1 H NMR (600MHz, DMSO-d 6 )δ14.05(d,J=86.0Hz,1H),9.73(d,J=288.3Hz,1H),8.32-8.20(m,1H),7.97(ddd,J=1...
Embodiment 2
[0050] Accurately weigh 1.5 mmol of dichloro-bis(dimethylsulfoxide) platinum (II) and 1.0 mmol of ligand ClClBC, dissolve dichloro-bis(dimethyl sulfoxide) platinum (II) In 1 mL of dimethyl sulfoxide solution, the ligand ClClBC was dissolved in 4 mL of methanol, and then the two solutions were mixed and put into a polytetrafluoroethylene reactor, and reacted at 100 ° C for 24 hours, and the program was set at 10 ° C / The temperature was lowered at a rate of h to room temperature, and a solid was precipitated. The solid was washed with distilled water, methanol, and ether in sequence, and complex 2 was obtained after vacuum drying with a yield of 85.3%.
[0051] The obtained complex 2 is identified:
[0052] (1) Infrared spectrum, its spectrogram is as follows Figure 8 Shown:
[0053] IR(KBr):3225,1729,1564,1407,1152,1111,1024,992,764,737,548,434cm -1 .
[0054] (2) Proton NMR spectrogram, its spectrogram is as Figure 5 shown.
[0055] 1 H NMR (600MHz, DMSO-d 6 )δ14.0...
Embodiment 3
[0063] Accurately weigh the amount of the substance as 2.0mmol dichlorobis(dimethylsulfoxide)platinum(II) and 1.0mmol ligand BrBrBC, dissolve dichlorobis(dimethylsulfoxide)platinum(II) In 0.5 mL of dimethyl sulfoxide solution, dissolve the ligand BrBrBC in 3 mL of methanol and 1 mL of distilled water, then mix the two solutions into a polytetrafluoroethylene reactor, react at 100 °C for 48 hours, and cool After reaching room temperature, yellow blocky solids were precipitated, which were washed with methanol and diethyl ether in sequence, and complex 3 was obtained after vacuum drying with a yield of 92.26%.
[0064] The resulting yellow granular product is identified:
[0065] (1) Infrared spectrum, its spectrogram is as follows Figure 12 Shown:
[0066] IR(KBr):3206,1727,1614,1555,1448,1401,1247,1150,1110,1023,975,935,740,528,432cm -1 .
[0067] (2) Proton NMR spectrogram, its spectrogram is as Figure 9 shown.
[0068] 1 H NMR (600MHz, DMSO-d 6 )δ14.02(d, J=82.0Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com