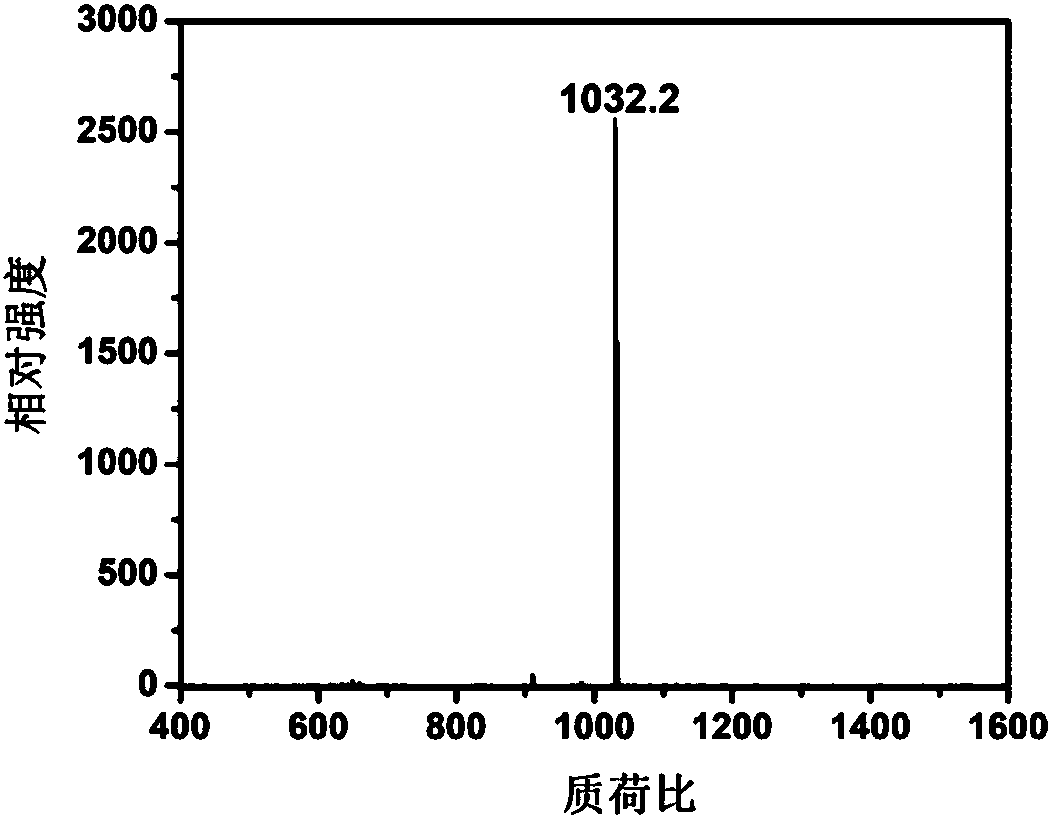
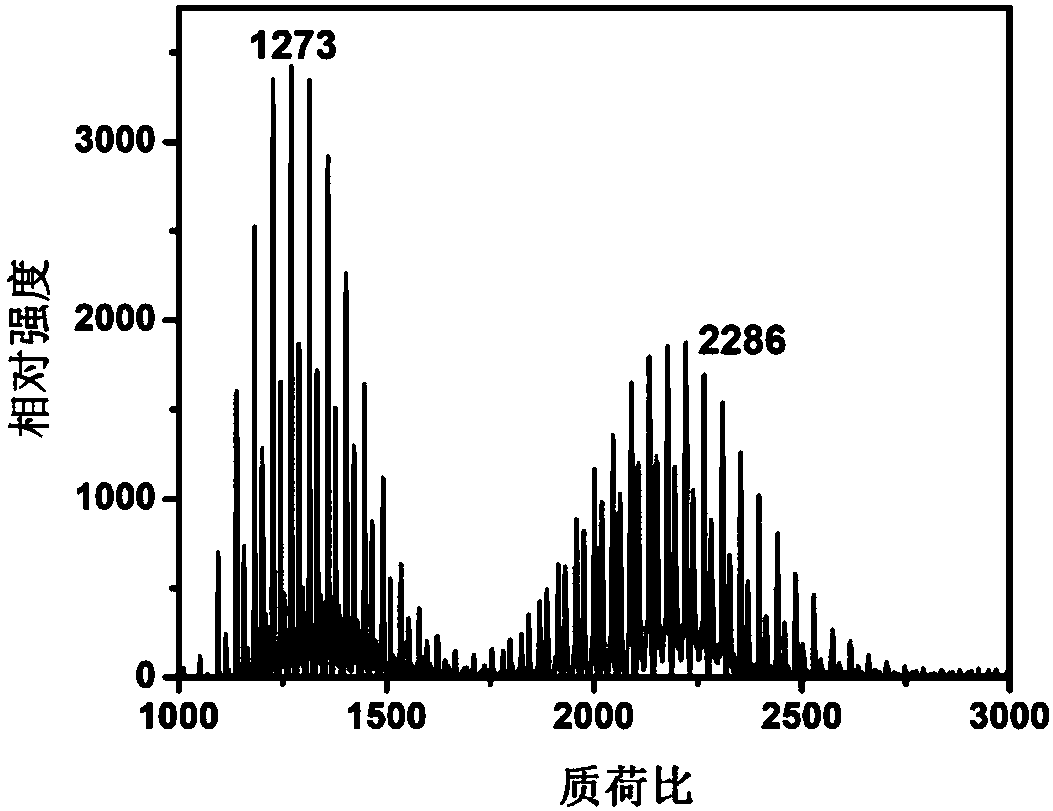
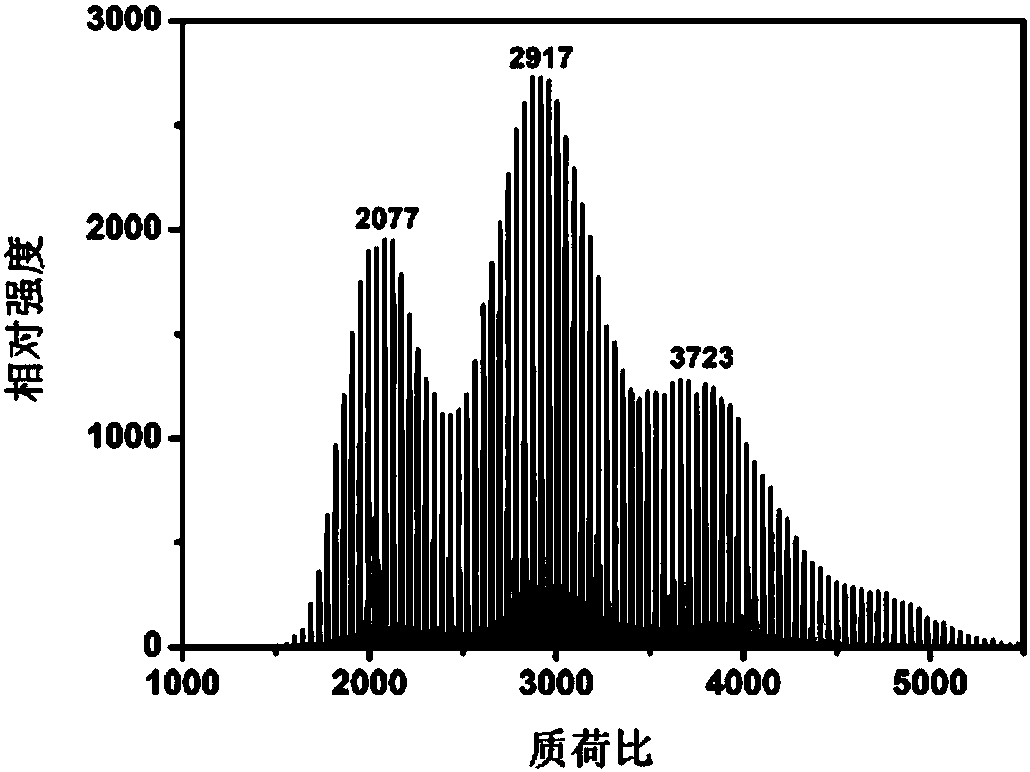
Fullerene phthalocyanine derivative as well as preparation method and application thereof
A technology of fullerene phthalocyanine derivatives and derivatives, applied in the field of fullerene phthalocyanine derivatives and their preparation, can solve the problems of low solubility, easy photodegradation, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] When the fullerene is C 70 , M is zinc (Zn), and when n=15, the fullerene phthalocyanine derivatives are as shown in formula 1-1, and its preparation method comprises:
[0082] (1) Add 0.12 millimolar C 70 , 1.4 millimoles of N-ethylglycine (purchased in Alfa; as reaction raw material), 1.4 millimoles of p-Hydroxybenzaldehyde (purchased in Alfa; as reaction raw material) are dissolved in 150 ml of toluene solution (purchased in Bailingwei; As a solvent), protected by an argon atmosphere, stirred and reacted at 110°C for 6 hours, removed most of the solvent by rotary evaporation, and separated through a silica gel column to obtain the compound shown in formula 4-1. Compound and 10 microliters of N,N-dimethylformamide (purchased from Bailingwei; as a catalyst) were added to 20 milliliters of anhydrous dichloromethane (purchased from Bailingwei; as a solvent), and at 0°C, 2.3 millimerol was added Acyl chloride (purchased from Enoch; as an acylating reagent), after stirri...
Embodiment 2
[0097] The fullerene phthalocyanine derivatives shown in the formula 1-1 obtained in Example 1 were tested for photodegradation stability, and the steps and results were as follows:
[0098] (1) DMSO solution (a) of fullerene phthalocyanine derivatives shown in formula 1-1 of preparation 10 micromole / liter embodiment 1 gained and tetracarboxylic acid phthalocyanine shown in 10 micromole / liter formula 2-1 The DMSO solution (b) of cyanine was placed in 650 nm red light for different time (0, 10, 20, 30, 40, 50, 60, 90 minutes), and its UV-Vis absorption spectrum and UV-Vis absorption spectrum were measured. The characteristic absorption intensity at 685nm in the absorption spectrum varies with the illumination time, and the results are shown in Figure 5(a), Figure 5(b) and Figure 5(c), respectively.
[0099] With the prolongation of the illumination time, if the change of the UV-visible absorption intensity is smaller, the sample is more stable and the anti-photobleaching abilit...
Embodiment 3
[0101] The fullerene phthalocyanine derivatives shown in the formula 1-1 obtained in Example 1 were tested for dark toxicity. The steps and results are as follows:
[0102] (1) Resuscitate Hela cells (Cell Resource Center, Shanghai Institute of Biology, Chinese Academy of Sciences), and adjust the cell density to 5×10 when the cells enter the logarithmic phase and grow stably. 4 / ml, seeded into 96-well plate, 37°C, 5% CO 2 , incubated for 24 hours;
[0103] (2) After the cells adhere to the wall, replace the DMEM solution (Corning R10-013-CV, purchasing company: Baierdi) with 200 microliters of concentrations of 0 (as a control), 4.5, 9, 13.5, 18, and 22.5 , the DMEM solution of the fullerene phthalocyanine derivatives obtained in embodiment 1 of 27 micromole / liter, 37 ℃, 5%CO 2 , incubate for 24 hours in the dark;
[0104] (3) Discard the DMEM solution containing the fullerene phthalocyanine derivatives obtained in Example 1, add a colorless DMEM solution of 10% CCK-8 (Do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com