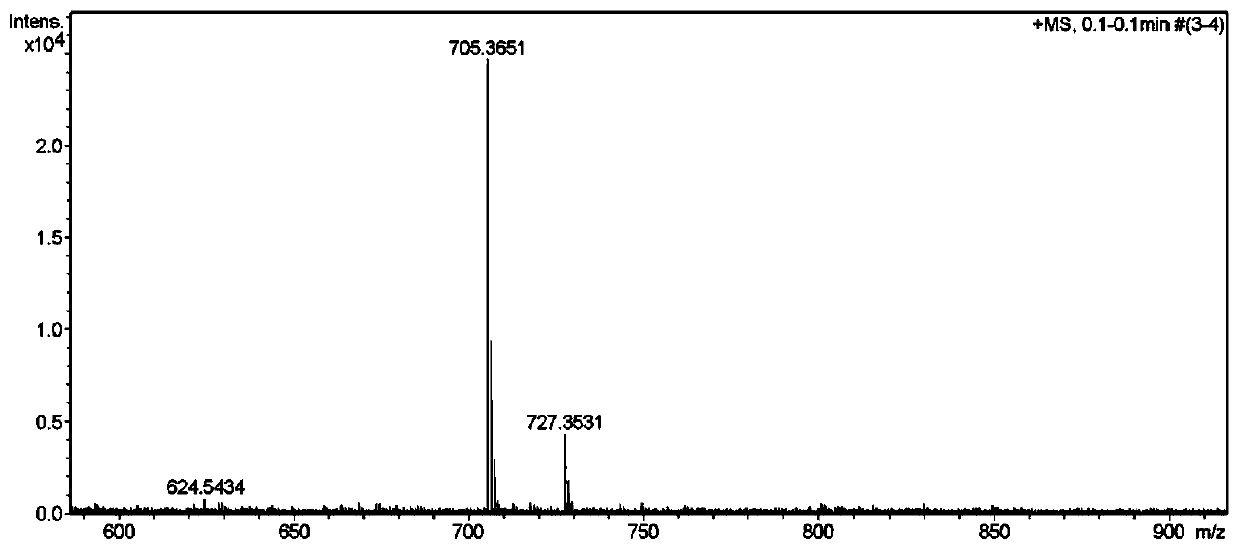
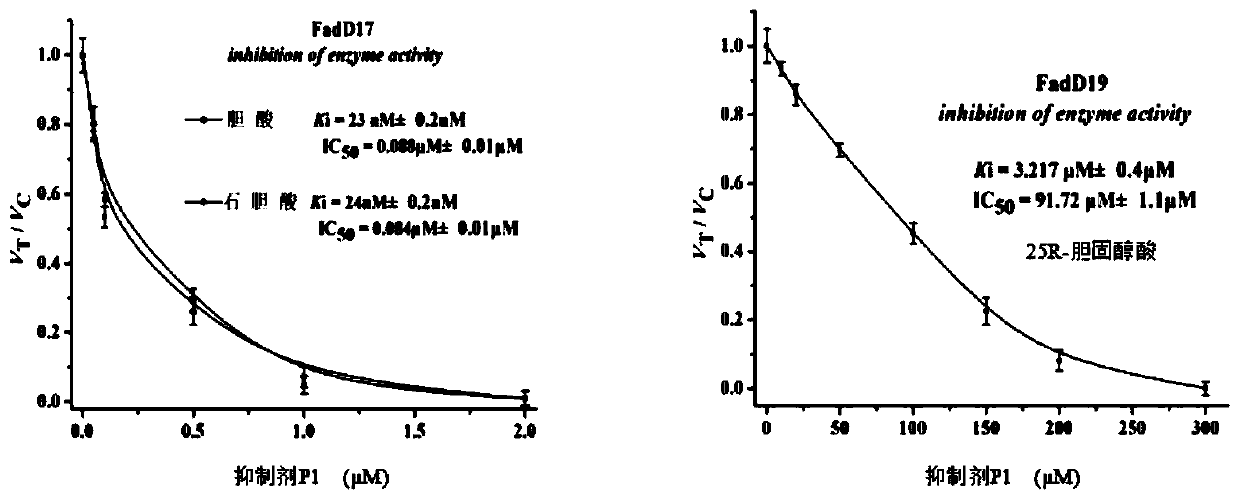
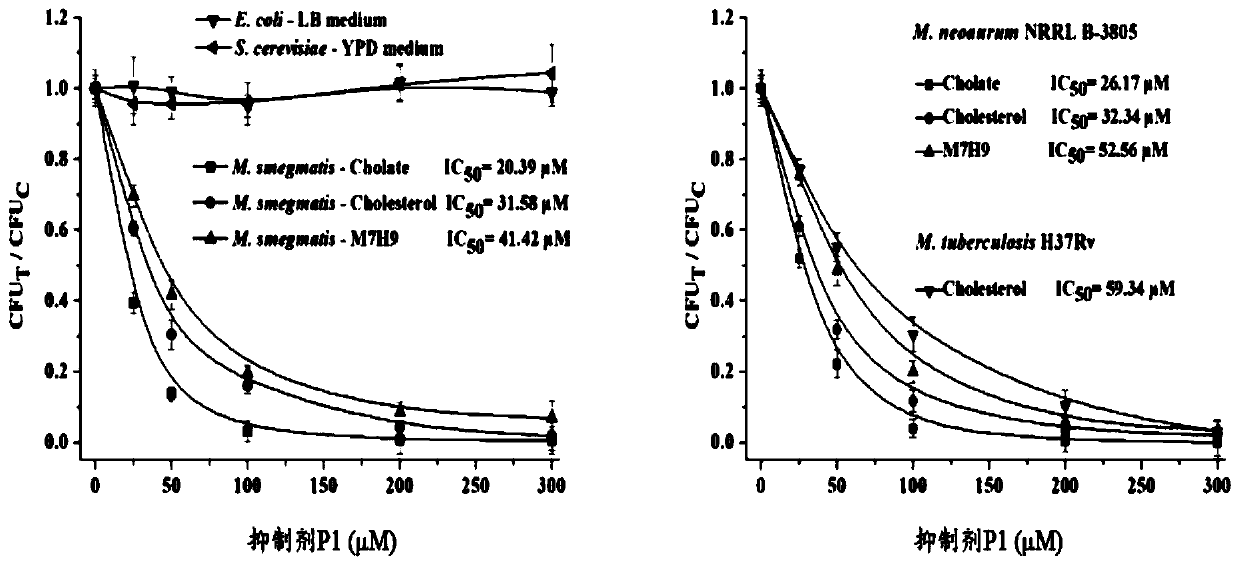
Synthetic method and application of a class of novel mycobacterial inhibitors
A technology for mycobacteria and uses, applied in the field of medicine, can solve problems such as no compounds that inhibit ester acyl-CoA synthase, and achieve the effect of good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of embodiment 1 inhibitor P1 (formula II)
[0035] synthetic route:
[0036]
[0037] The preparation method of compound 5, it comprises the following steps:
[0038] Step 1: Under the protection of argon, add adenosine (10.0g), DMF (40mL), imidazole (22.0g) and tert-butyldimethylsilyl chloride (50g) into a round bottom bottle in turn, at 40 degrees Celsius The reaction was stopped after stirring for 13 h. Dichloromethane was added for extraction, and the organic layer was sequentially washed with saturated NaHCO 3 、H 2 O was washed several times, dried and filtered, concentrated under reduced pressure, and column chromatography gave light yellow oily liquid compound 1.
[0039] Step 2: Weigh compound 1 (10g), 80% CH 3 COOH (100mL) was placed in a round-bottomed flask, refluxed at 100°C for 5h, then stopped the reaction, adjusted the pH to 8 with saturated NaOH, extracted with ethyl acetate, washed the organic layer several times with wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com