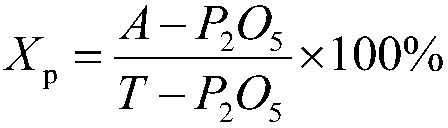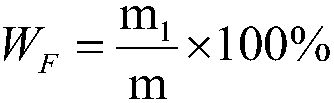Method for decomposing phosphorite through urea nitrate to prepare granular urea nitrate compound fertilizer
A technology of nitro compound fertilizer and compound fertilizer, which is applied in the directions of nitrate fertilizer, urea compound fertilizer, phosphate fertilizer, etc., can solve the problems of affecting fertilizer nutrients, declining economic benefits, calcium polyphosphate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
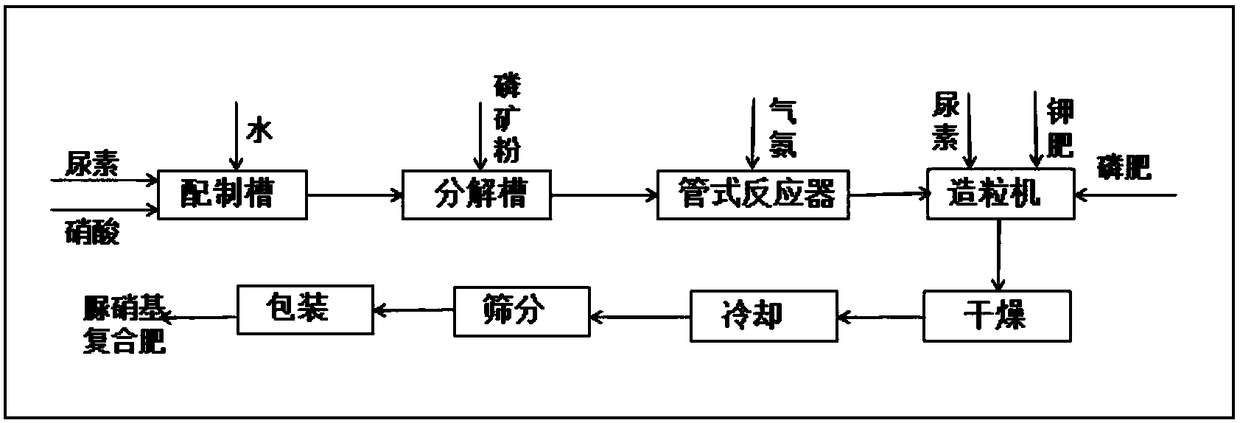
[0073] Embodiment 1: Decompose phosphate rock with urea nitric acid to prepare granular urea nitro compound fertilizer
[0074] The implementation steps of this embodiment are as follows:
[0075] A, preparation of urea nitric acid aqueous solution
[0076] Solid urea, nitric acid with a concentration of 45% by weight and water are mixed and dissolved in a molar ratio of 1.6:1.0:4.0 to obtain an aqueous solution of urea nitric acid;
[0077] B. Decomposition of phosphate rock
[0078] According to the weight ratio of urea nitric acid aqueous solution and phosphate rock powder 4.0:1.0, in the urea nitric acid aqueous solution that step A obtains, add particle size and be-100 order (150 μ m) reaches 95.2%, P 2 o 5 Content is phosphate rock powder of 25% by weight, then reacted at a temperature of 28° C. for 60 minutes to obtain an acidic slurry; adopt NY / T 1973-2010 Water-soluble Fertilizer Water-insoluble Content and pH Determination Method Detection, said The pH of the aci...
Embodiment 2
[0084] Embodiment 2: Decompose phosphate rock with urea nitric acid to prepare granular urea nitro compound fertilizer
[0085] The implementation steps of this embodiment are as follows:
[0086] A, preparation of urea nitric acid aqueous solution
[0087] Solid urea, nitric acid with a concentration of 50% by weight and water are mixed and dissolved in a molar ratio of 0.3:1.0:4.2 to obtain an aqueous solution of urea nitric acid;
[0088] B. Decomposition of phosphate rock
[0089] According to the weight ratio of urea nitric acid aqueous solution and phosphate rock powder 2.8:1.0, in the urea nitric acid aqueous solution that step A obtains, add particle size and be-100 order (150 μ m) reach 95.8%, P 2 o 5 Content is phosphate rock powder of 15% by weight, then reacted at a temperature of 20° C. for 30 minutes to obtain an acidic slurry; adopt NY / T 1973-2010 Water-soluble Fertilizer Water-insoluble Content and pH Determination Method Detection, said The pH of the acidi...
Embodiment 3
[0095] Embodiment 3: Decompose phosphate rock with urea nitric acid to prepare granular urea nitro compound fertilizer
[0096] The implementation steps of this embodiment are as follows:
[0097] A, preparation of urea nitric acid aqueous solution
[0098] Solid urea, nitric acid with a concentration of 48% by weight and water are mixed and dissolved according to the molar ratio of 2.0:1.0:4.4 to obtain an aqueous solution of urea nitric acid;
[0099] B. Decomposition of phosphate rock
[0100] According to the weight ratio of urea nitric acid aqueous solution and phosphate rock powder 3.2:1.0, in the urea nitric acid aqueous solution that step A obtains, add particle size and be-100 order (150 μ m) reaches 96.1%, P 2 o 5 Content is phosphate rock powder of 20% by weight, then reacted at a temperature of 45° C. for 15 minutes to obtain an acidic slurry; adopt NY / T 1973-2010 Water-soluble Fertilizer Water-insoluble Content and pH Determination Method Detection, said The p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com