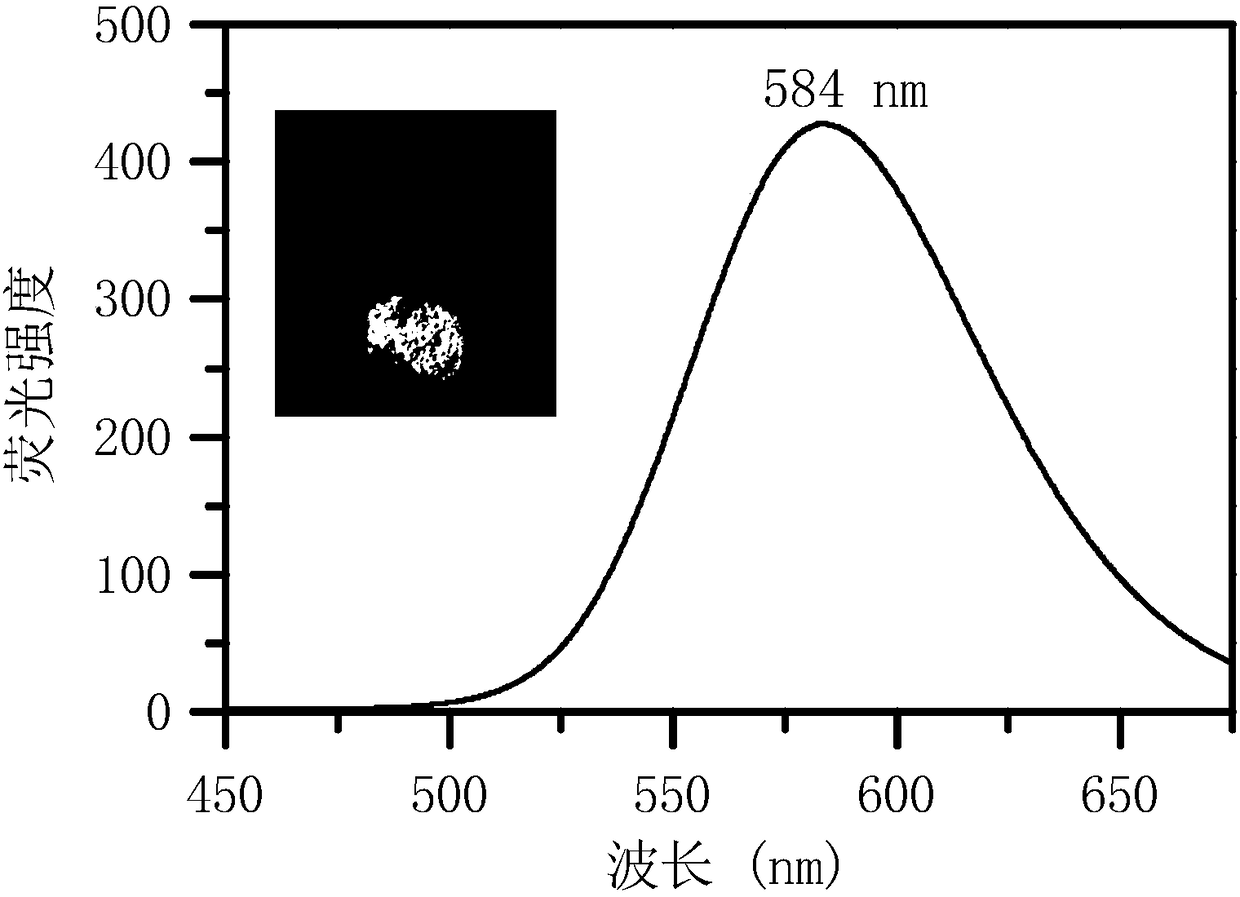
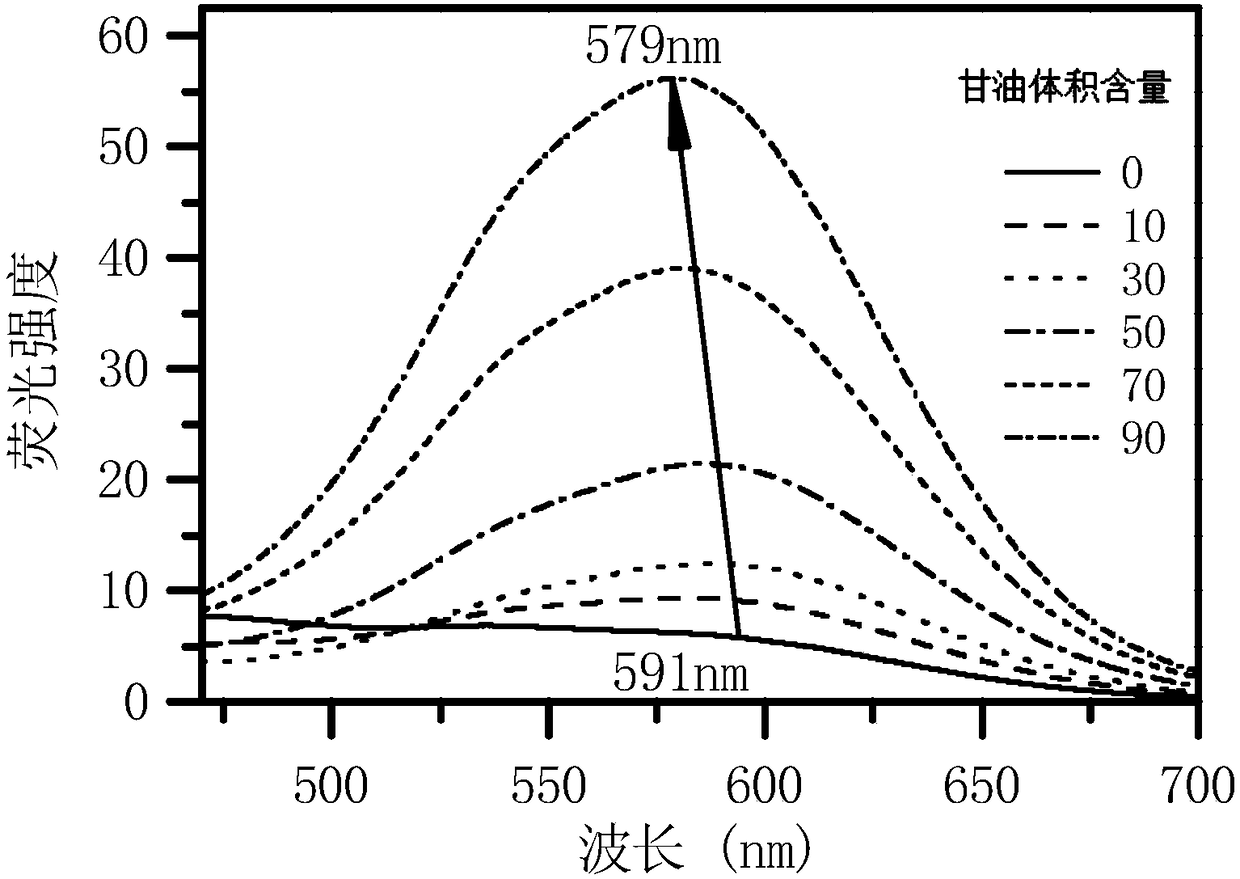
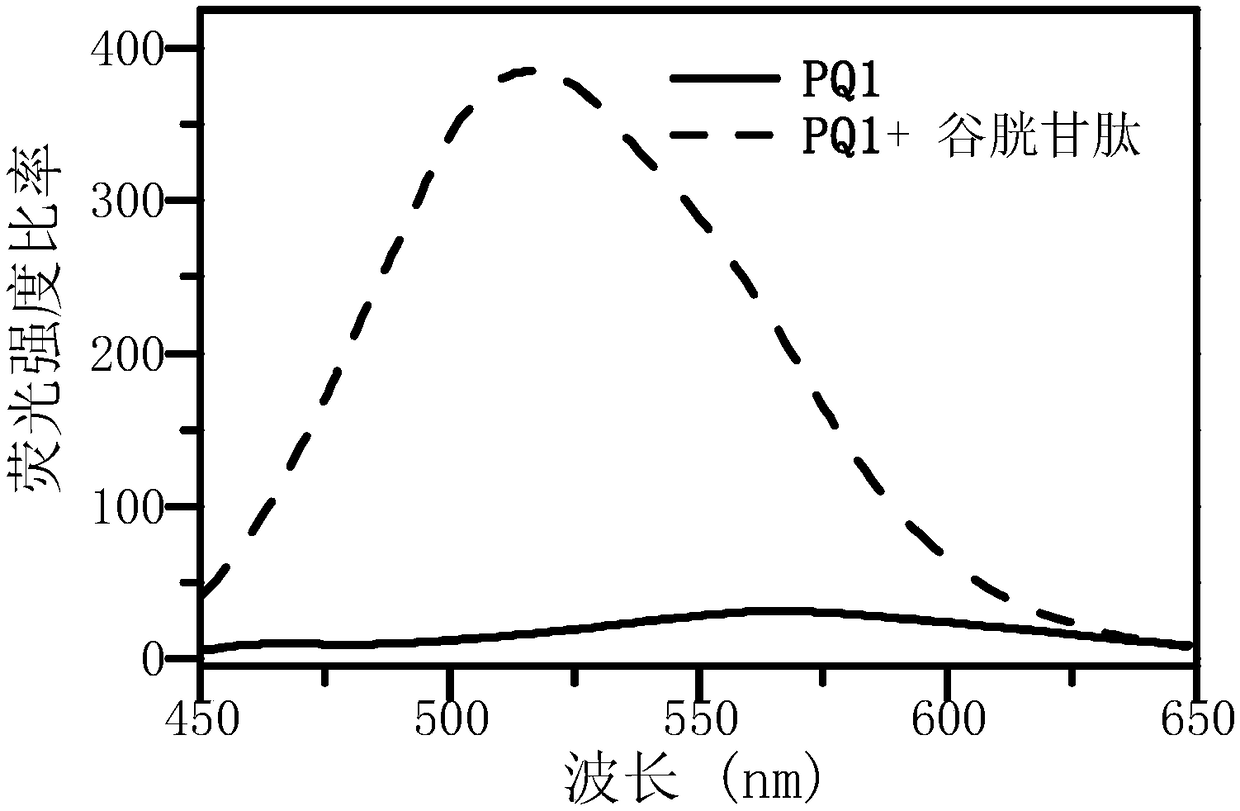
Water-soluble aggregation-induced emission quinoxaline compound, preparation method thereof and application of compound
A technology of aggregation-induced luminescence and quinoxaline, which can be applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., and can solve the problem of rare species of luminescent quinoxalines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Embodiment 1: Preparation of pyridinium salt quinoxaline compound PQ1
[0036] (1) Preparation of 5,8-dibromo-2,3-bis(4-methoxybenzene)quinoxaline according to literature H.J.Song, D.H.Kim, E.J.Lee, J.R.Haw, D.K.Moon, Sol.Energ.Mat. Prepared by the method disclosed in Sol.C.2014, 123, 112-121.
[0037] (2) Dissolve 10mmol of 5,8-dibromo-2,3-bis(4-methoxybenzene)quinoxaline and 10mmol triphenylethylene borate (0.45mM, 171.9mg) in 25mL of toluene- Mixed solvent of 5mL absolute ethanol-5mL sodium carbonate aqueous solution (1mol / L), after vacuuming and changing nitrogen three times, add 1.5mmol Pd(PPh 3 ) 4 , heated the reaction system to 95°C, stirred and refluxed for 12 hours, then spotted the plate by TLC to detect the completion of the reaction of the raw materials, stopped heating and cooled to room temperature, washed the reaction solution with water for 3 times, and then washed with CH 2 Cl 2 Extracted 3 times with anhydrous NaSO 4 Dry, filter, remove the solven...
Embodiment 2
[0041] Embodiment 2: prepare pyridinium salt quinoxaline compound PQ2
[0042] (1) Preparation of 5,8-dibromo-2,3-bis(4-methoxybenzene)quinoxaline according to literature H.J.Song, D.H.Kim, E.J.Lee, J.R.Haw, D.K.Moon, Sol.Energ.Mat. Prepared by the method disclosed in Sol.C.2014, 123, 112-121.
[0043] (2) Dissolve 10mmol of 5,8-dibromo-2,3-bis(4-methoxybenzene)quinoxaline and 10mmol triphenylethylene borate (0.45mM, 171.9mg) in 25mL of toluene- Mixed solvent of 5mL absolute ethanol-5mL sodium carbonate aqueous solution (1mol / L), after vacuuming and changing nitrogen three times, add 0.8mmol Pd(PPh 3 ) 4 , heated the reaction system to 95°C, stirred and refluxed for 12 hours, then spotted the plate by TLC to detect the completion of the reaction of the raw materials, stopped heating and cooled to room temperature, washed the reaction solution with water for 3 times, and then washed with CH 2 Cl 2 Extracted 3 times with anhydrous NaSO 4 Dry, filter, remove the solvent by r...
Embodiment 3
[0046] Embodiment 3: prepare pyridinium salt quinoxaline compound PQ3
[0047] (1) Preparation of 5,8-dibromo-2,3-bisphenylquinoxaline according to literature Y.Chen, Y.Ling, L.Ding, C.Xiang and G.Zhou, J.Mater.Chem.C. 2016, 4:4 (2016) 8496-8505 disclosed method preparation.
[0048] (2) Dissolve 10mmol of 5,8-dibromo-2,3-bisphenylquinoxaline and 10mmol triphenylethylene borate (0.45mM, 171.9mg) in 25mL toluene-5mL absolute ethanol-5mL Sodium carbonate aqueous solution (1mol / L) mixed solvent, after vacuumizing and changing nitrogen three times, add 1.5mmol Pd (PPh 3 ) 4 , heat the reaction system to 60°C, stir for 5 hours, then spot the plate by TLC to detect the completion of the reaction of the raw materials, stop heating and cool to room temperature, wash the reaction solution with water for 3 times, and then wash with CH 2 Cl 2 Extracted 3 times with anhydrous NaSO 4 Dry, filter, remove the solvent by rotary evaporation, and separate by silica gel column chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com