Method for preparing celecoxib nano-crystal preparation
A technology of celecoxib and nanocrystals, which is used in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of long preparation time, easy pollution, etc., and meets the requirements of equipment and personnel quality. The effect of low burden and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
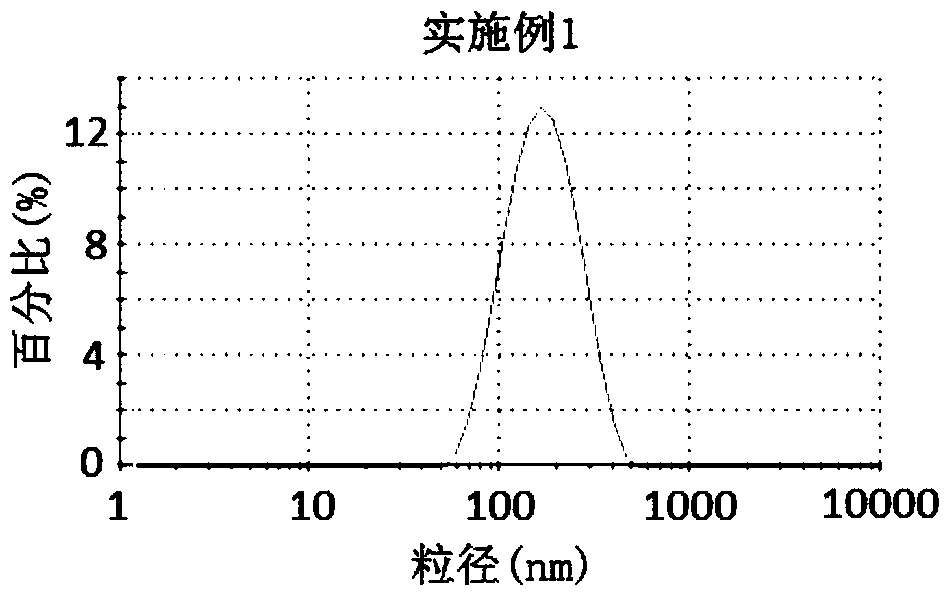
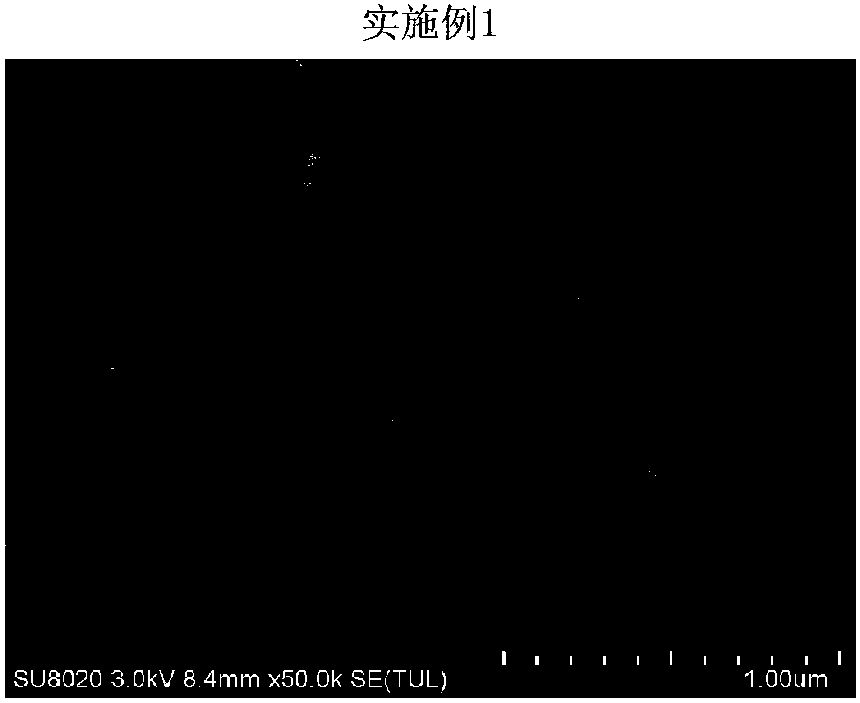
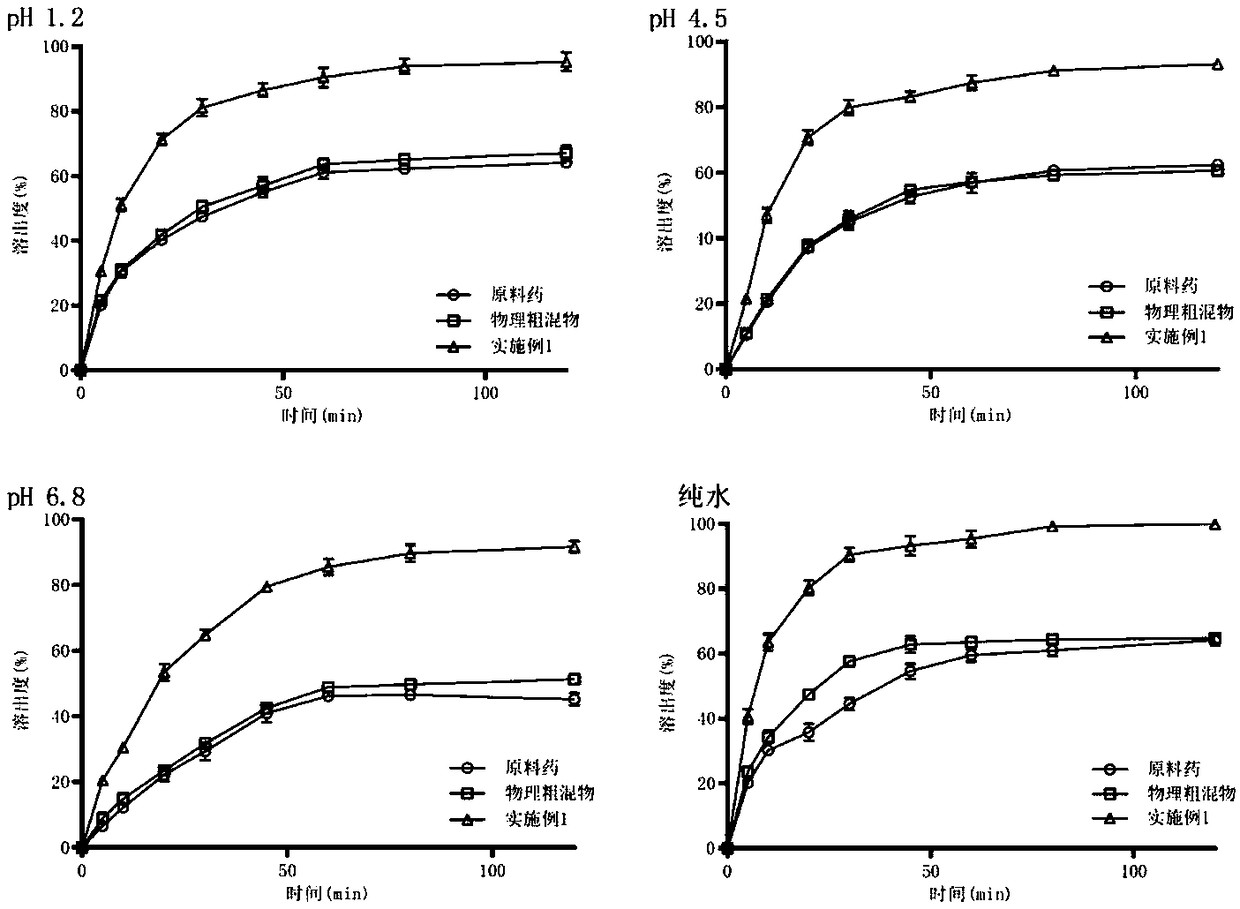
Embodiment 1
[0028] Weigh 4g of hydroxypropyl cellulose (HPC) and 0.5g of sodium dodecyl sulfate (SDS), add 500mL of pure water, and stir slowly for 30min until the solution is clear. Weigh 20 g of the celecoxib bulk drug, add the above aqueous solution under slow stirring, and disperse the bulk drug particles in the solution to obtain a coarse suspension. Add 250mL of grinding media (specification d=0.3mm) into the grinding chamber (500mL) of the sand mill, and feed the coarse suspension into the grinding chamber through a feed pump, and grind for 100min at a speed of 10m / s to obtain a nanosuspension. The obtained nanosuspension was freeze-dried at -55° C. for 10 h to obtain a celecoxib nanocrystal preparation.
Embodiment 2
[0030] Weigh 4g of hypromellose acetate succinate (HPMC-AS) and 0.5g of sodium dodecyl sulfate (SDS), add 500mL of pure water, and stir slowly for 30min until the solution is clear. Weigh 20 g of the celecoxib bulk drug, add the above aqueous solution under slow stirring, and disperse the bulk drug particles in the solution to obtain a coarse suspension. Add 250mL of grinding media (specification d=0.3mm) into the grinding chamber (500mL) of the sand mill, and feed the coarse suspension into the grinding chamber through a feed pump, and grind for 100min at a speed of 10m / s to obtain a nanosuspension. The obtained nanosuspension was freeze-dried at -55° C. for 10 h to obtain a celecoxib nanocrystal preparation.
Embodiment 3
[0032] Weigh 4g of polyvinylpyrrolidone (PVP K30) and 0.5g of sodium dodecyl sulfate (SDS), add 500mL of pure water, and stir slowly for 30min until the solution is clear. Weigh 20 g of the celecoxib bulk drug, add the above aqueous solution under slow stirring, and disperse the bulk drug particles in the solution to obtain a coarse suspension. Add 250mL of grinding media (specification d=0.3mm) into the grinding chamber (500mL) of the sand mill, and feed the coarse suspension into the grinding chamber through a feed pump, and grind for 100min at a speed of 10m / s to obtain a nanosuspension. The obtained nanosuspension was freeze-dried at -55° C. for 10 h to obtain a celecoxib nanocrystal preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com