Method for improving expression of selenoprotein TrxR
A technology of selenoproteins and culturing strains, which is applied in the field of improving the expression of selenoprotein TrxR, can solve the problems affecting the separation and purification of selenoproteins, restricting the research of selenoproteins, and the low expression of selenoproteins. The method is simple, the raw materials are easy to obtain, and the cost is low. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
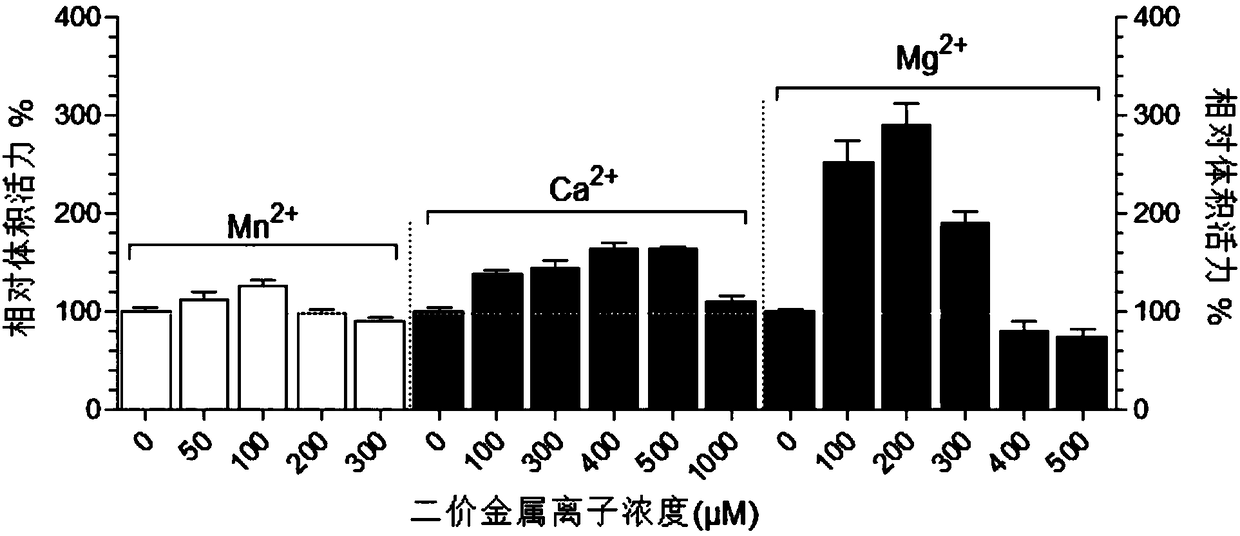
[0024] Use 250ml glass Erlenmeyer shaker flask experiment, add 50ml LB medium, 50μl 15mg / ml tetracycline, 50μl 50mg / ml kanamycin and 50μl 34mg / ml chloramphenicol, 1ml inoculum. Use a shaker to culture at 37°C and 220rpm until the end of logarithmic growth, add 50μl of 0.5M IPTG, 50μl of 5mM selenite, 50μl of 100mg / ml L-cysteine and 2.5-15μl of 1M MnCl 2 , and then low temperature induced expression at 24°C for 24h. Take out 1ml of the bacterial solution, centrifuge to discard the supernatant, add 50μl TE buffer, 1μl 1mg / ml lysozyme, freeze and thaw 3 times repeatedly to dissolve the broken bacterial protein, centrifuge at 13,000rpm for 10min, collect the supernatant as the crude enzyme containing TrxR liquid. Use the DTNB method to test the activity, take the enzyme volume activity as the index, and find that Mn 2+ The expression of TrxR can be promoted in a certain concentration range. When Mn 2+ When the concentration is 100μM, the effect of promoting expression is bet...
Embodiment 2
[0026] Use 250ml glass Erlenmeyer shaker flask experiment, add 50ml LB medium, 50μl 15mg / ml tetracycline, 50μl 50mg / ml kanamycin and 50μl 34mg / ml chloramphenicol, 1ml inoculum. Use a shaker to culture at 37°C and 220rpm until the end of logarithmic growth, add 50μl of 0.5M IPTG, 50μl of 5mM selenite, 50μl of 100mg / ml L-cysteine and 5-50μl of 1M CaCl 2 , and then low temperature induced expression at 24°C for 24h. Take out 1ml of the bacterial solution, centrifuge to discard the supernatant, add 50μl TE buffer, 1μl 1mg / ml lysozyme, freeze and thaw 3 times repeatedly to dissolve the broken bacterial protein, centrifuge at 13,000rpm for 10min, collect the supernatant as the crude enzyme containing TrxR liquid. Use the DTNB method to test the activity, take the enzyme volume activity as the index, and find that Ca 2+ The expression of TrxR can be promoted in a certain concentration range. When Ca 2+ When the concentration is 400μM, the effect of promoting expression is bette...
Embodiment 3
[0028] Use 250ml glass Erlenmeyer shaker flask experiment, add 50ml LB medium, 50μl 15mg / ml tetracycline, 50μl 50mg / ml kanamycin and 50μl 34mg / ml chloramphenicol, 1ml inoculum. Use a shaker to culture at 37°C and 220rpm until the end of logarithmic growth, add 50μl of 0.5M IPTG, 50μl of 5mM selenite, 50μl of 100mg / ml L-cysteine and 5-25μl of 1M MgCl 2 , and then low temperature induced expression at 24°C for 24h. Take out 1ml of the bacterial solution, centrifuge to discard the supernatant, add 50μl TE buffer, 1μl 1mg / ml lysozyme, freeze and thaw 3 times repeatedly to dissolve the broken bacterial protein, centrifuge at 13,000rpm for 10min, collect the supernatant as the crude enzyme containing TrxR liquid. Use the DTNB method to test the activity, take the enzyme volume activity as the index, and find that Mg 2+ The expression of TrxR can be promoted in a certain concentration range. When Mg 2+ When the concentration is 300μM, the effect of promoting expression is bette...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com