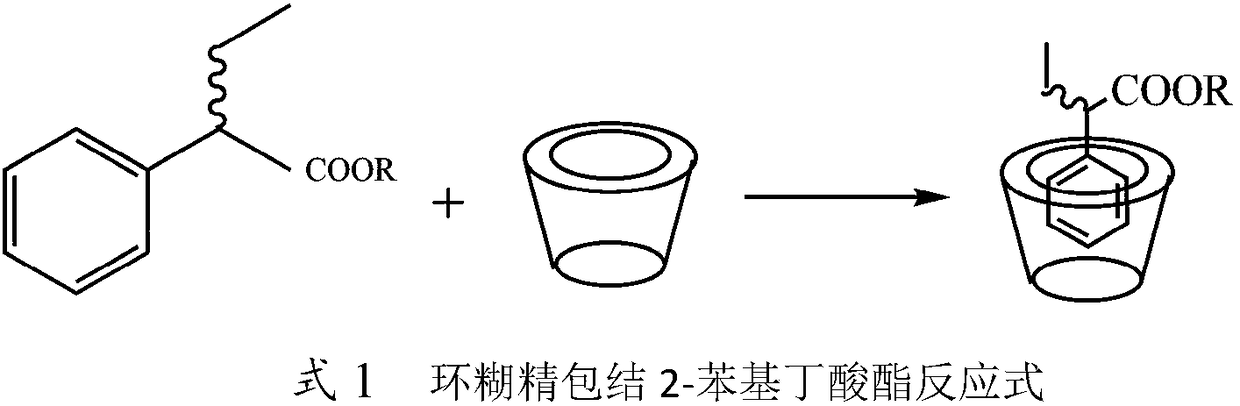
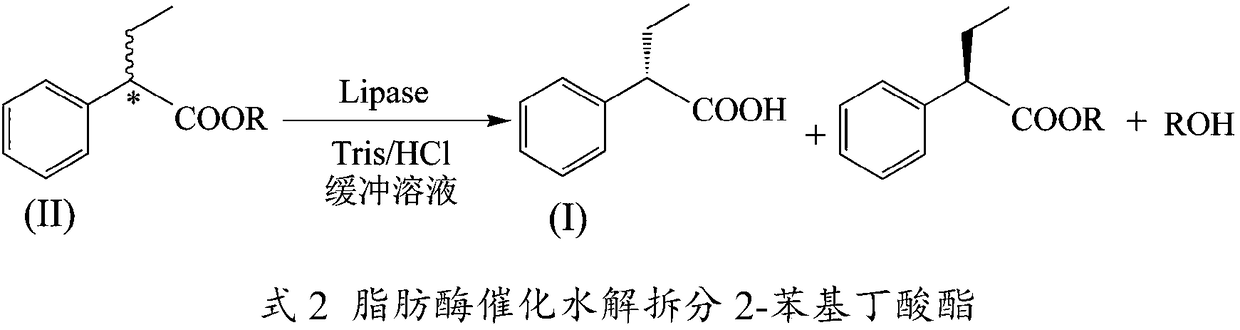
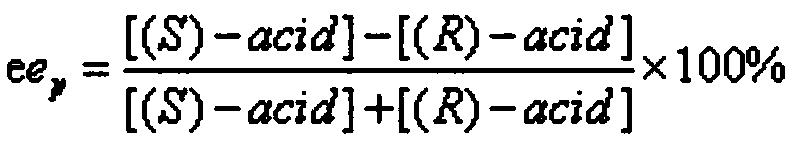Method for preparing (S)-2-phenylbutyric acid by stereoselective enzyme catalytic hydrolysis
A technology of stereoselectivity and phenylbutyric acid, applied in fermentation and other fields, can solve the problems of 2-phenylbutyric acid, such as low optical purity, environmental pollution, complicated operation, etc., and achieve the goal of overcoming toxicity, simple operation, and improving conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 25mL reaction tube, add 0.020mmol of racemic ethyl 2-phenylbutyrate as the substrate, 1mL of Tris / hydrochloric acid buffer solution (pH=6) as the reaction medium, add 10mg of different Commercial lipase was used to start the reaction, and the reaction was carried out at 600rpm and 50°C for 5h. After the reaction, the product was analyzed by high performance liquid chromatography. The results showed that: when Candida antarctica lipase A was used as a catalyst, it preferentially recognized (S)-2-phenylbutyric acid ethyl ester, and its ee p is 64.46%, c is 5.45%, and E is 4.8.
Embodiment 2
[0031] In a 25mL reaction tube, add 0.015mmol of racemic 2-phenylbutyrate as the substrate, 1mL of tris / hydrochloric acid buffer solution (pH=6) as the reaction medium, and add 30mg of Antarctic Candida Yeast lipase A, start the reaction, and react for 4 hours at 600rpm and 85°C. After the reaction, the product was analyzed by high performance liquid chromatography. The results showed that when 2-phenylbutyrate heptyl was used as the substrate, its ee p is 96.74%, c is 15.44%, and E is 71.75.
Embodiment 3
[0033] In a 25mL reaction tube, add 0.015mmol of racemic 2-phenylhexylbutyrate as the substrate, 1mL of Tris / hydrochloric acid buffer solution (pH=6) as the reaction medium, add 30mg of Antarctica Trichotrichum lipase A, start the reaction, and react for 4 hours at 600 rpm and 85°C. After the reaction, the product was analyzed by high performance liquid chromatography. The results show that: its ee p is 96.7%, c is 12.30%, and E is 68.10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com