A kind of enteric-coated tablet containing s-carboxymethyl-l-cysteine
A technology of cystine injection and cysteine, which is applied in the direction of medical preparations containing active ingredients, pill delivery, and medical preparations with non-active ingredients. Improve bioavailability, easy storage, and reduce irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
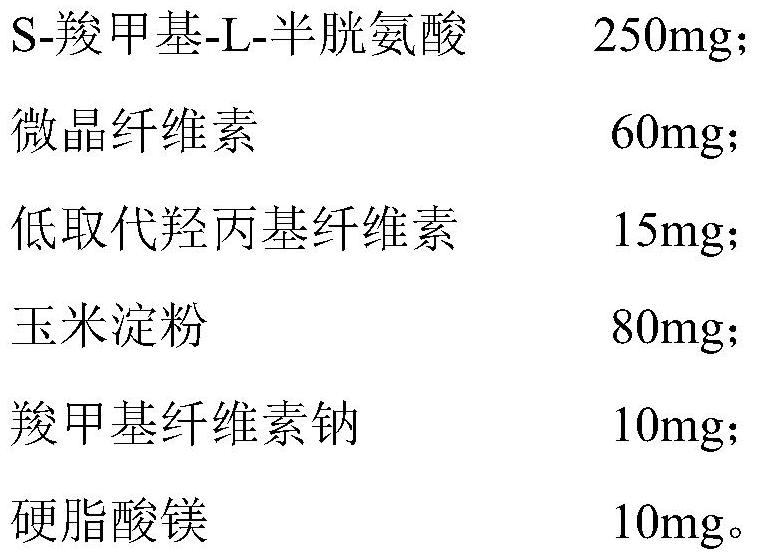
[0048] Chip
[0049]
[0050] Isolation layer
[0051] Hypromellose 6mg;
[0052] Enteric layer
[0053] Waterborne acrylic enteric coating system 93A 36.5mg;
[0054] Simethicone 0.37mg;
[0055] PEG8000 2.5 mg.
[0056] Preparation method:
[0057] Weigh the prescribed amount of tablet core materials, mix them evenly, pass through a 100-mesh sieve, make a soft material with water, pass through a 24-mesh sieve, dry the obtained wet granules, and press into tablets. Weigh the prescribed amount of hypromellose, and disperse it with the prescribed amount of purified water to obtain the isolation layer. The enteric-coated layer material is weighed and dispersed with purified water to obtain the enteric-coated layer. Wrap the S-carboxymethyl-L-cysteine tablet with the isolation layer and the enteric-coated layer in turn to obtain the S-carboxymethyl-L-cysteine enteric-coated tablet.
Embodiment 2
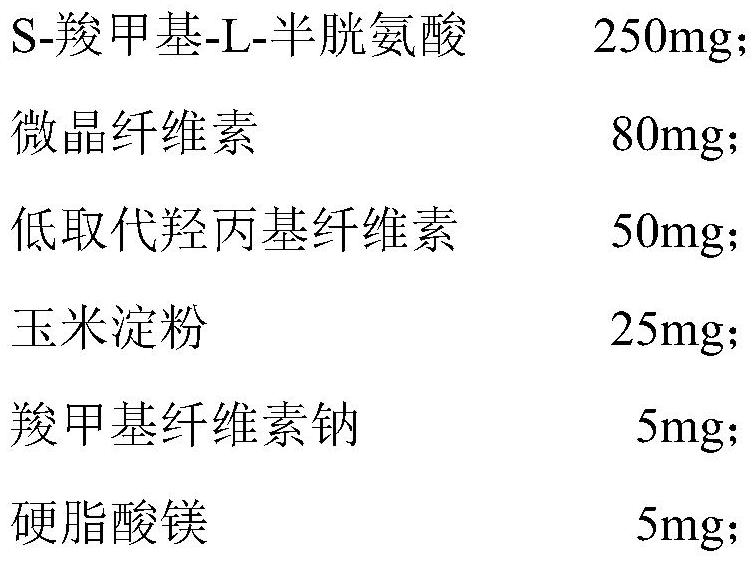
[0059] Chip
[0060]
[0061] Isolation layer
[0062] Hypromellose 4mg;
[0063] Enteric layer
[0064] Waterborne acrylic enteric coating system 93A 73mg;
[0065] Simethicone 0.73mg;
[0066] Glyceryl Triacetate 5mg.
[0067] Preparation method:
[0068] Weigh the prescribed amount of tablet core materials, mix them evenly, pass through a 100-mesh sieve, make a soft material with water, pass through a 24-mesh sieve, dry the obtained wet granules, and press into tablets. Weigh the prescribed amount of hypromellose, and disperse it with the prescribed amount of purified water to obtain the isolation layer. The enteric-coated layer material is weighed and dispersed with purified water to obtain the enteric-coated layer. Wrap the S-carboxymethyl-L-cysteine tablet with the isolation layer and the enteric-coated layer in turn to obtain the S-carboxymethyl-L-cysteine enteric-coated tablet.
Embodiment 3
[0070] Chip
[0071]
[0072] Isolation layer
[0073] Hypromellose 16mg;
[0074] Enteric layer
[0075] Waterborne acrylic enteric coating system 93A 73mg;
[0076] Simethicone 1mg;
[0077] Triethyl Citrate 10mg.
[0078] Preparation method:
[0079] Weigh the prescribed amount of tablet core materials, mix them evenly, pass through a 100-mesh sieve, make a soft material with water, pass through a 24-mesh sieve, dry the obtained wet granules, and press into tablets. Weigh the prescribed amount of hypromellose, and disperse it with the prescribed amount of purified water to obtain the isolation layer. The enteric-coated layer material is weighed and dispersed with purified water to obtain the enteric-coated layer. Wrap the S-carboxymethyl-L-cysteine tablet with the isolation layer and the enteric-coated layer in turn to obtain the S-carboxymethyl-L-cysteine enteric-coated tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com