Injection type bone filling material and preparation method and using method thereof
A filling material and injection-type technology, which is applied in the field of medical materials, can solve the problems of pain and inflammation of patients, and achieve the effects of strong solubility and dissolution, avoiding re-injury, and improving utilization and efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
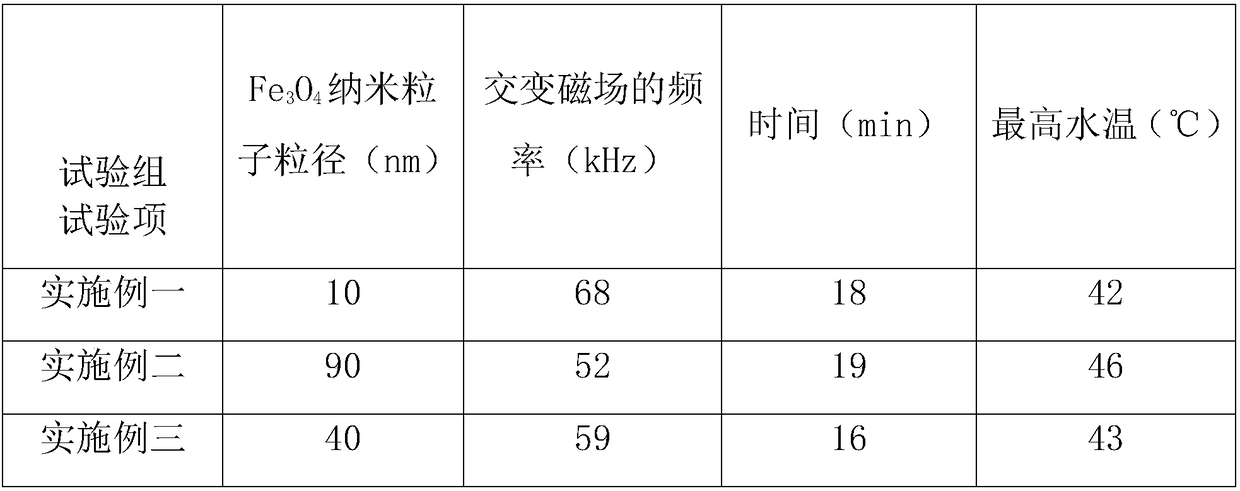
[0025] An injectable bone filling material, comprising β-tricalcium phosphate bone cement, solidification solution and drug-loaded microspheres, wherein the mass percentage of the drug-loaded microspheres is 6 wt%, and the particle size of the drug-loaded microspheres is 40-150nm, The solidification solution comprises the following components by mass percentage: 0.5wt% carboxymethyl chitosan, 8wt% citric acid, 1wt% polyvinyl alcohol, and the balance is deionized water; Fe 3 o 4 Nanoparticles are the core material, and a core-shell structure of poly N-isopropylacrylamide hydrogel is compounded on the surface of the core material, Fe 3 o 4 The surface of the nanoparticles is modified with hydroxypropyl-β-cyclodextrin, the drug is loaded on the hydroxypropyl-β-cyclodextrin, and the drug is imipenem. Its preparation method is as follows:
[0026] Using atom transfer radical method and basic ring-opening method on Fe 3 o 4 Hydroxypropyl-β-cyclodextrin is modified on the surfa...
Embodiment 2
[0028]An injectable bone filling material, comprising β-tricalcium phosphate bone cement, solidification solution and drug-loaded microspheres, wherein the mass percentage of the drug-loaded microspheres is 12wt%, and the particle diameter of the drug-loaded microspheres is 40-150nm. The solution includes the following components by mass percentage: 1.8wt% carboxymethyl chitosan, 15wt% citric acid, 2wt% polyvinyl alcohol, and the balance is deionized water; Fe 3 o 4 Nanoparticles are the core material, and a core-shell structure of poly N-isopropylacrylamide hydrogel is compounded on the surface of the core material, Fe 3 o 4 The surface of the nanoparticles is modified with hydroxypropyl-β-cyclodextrin, the drug is loaded on the hydroxypropyl-β-cyclodextrin, and the drug is a blend of resveratrol and levofloxacin. Its preparation method is as follows:
[0029] Using atom transfer radical method and basic ring-opening method on Fe 3 o 4 Hydroxypropyl-β-cyclodextrin is mo...
Embodiment 3
[0031] An injectable bone filling material, comprising β-tricalcium phosphate bone cement, solidification solution and drug-loaded microspheres, wherein the mass percentage of the drug-loaded microspheres is 10wt%, and the particle diameter of the drug-loaded microspheres is 40-150nm. The liquid comprises the following components by mass percentage: carboxymethyl chitosan 1.2wt%, citric acid 9wt%, polyvinyl alcohol 1.5wt%, and the balance is deionized water; Fe 3 o 4 Nanoparticles are the core material, and a core-shell structure of poly N-isopropylacrylamide hydrogel is compounded on the surface of the core material, Fe 3 o 4 The surface of the nanoparticles is modified with hydroxypropyl-β-cyclodextrin, the drug is loaded on the hydroxypropyl-β-cyclodextrin, and the drug is a blend of ciprofloxacin and sulbactam. Its preparation method is as follows:
[0032] Using atom transfer radical method and basic ring-opening method on Fe 3 o 4 Hydroxypropyl-β-cyclodextrin is mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com