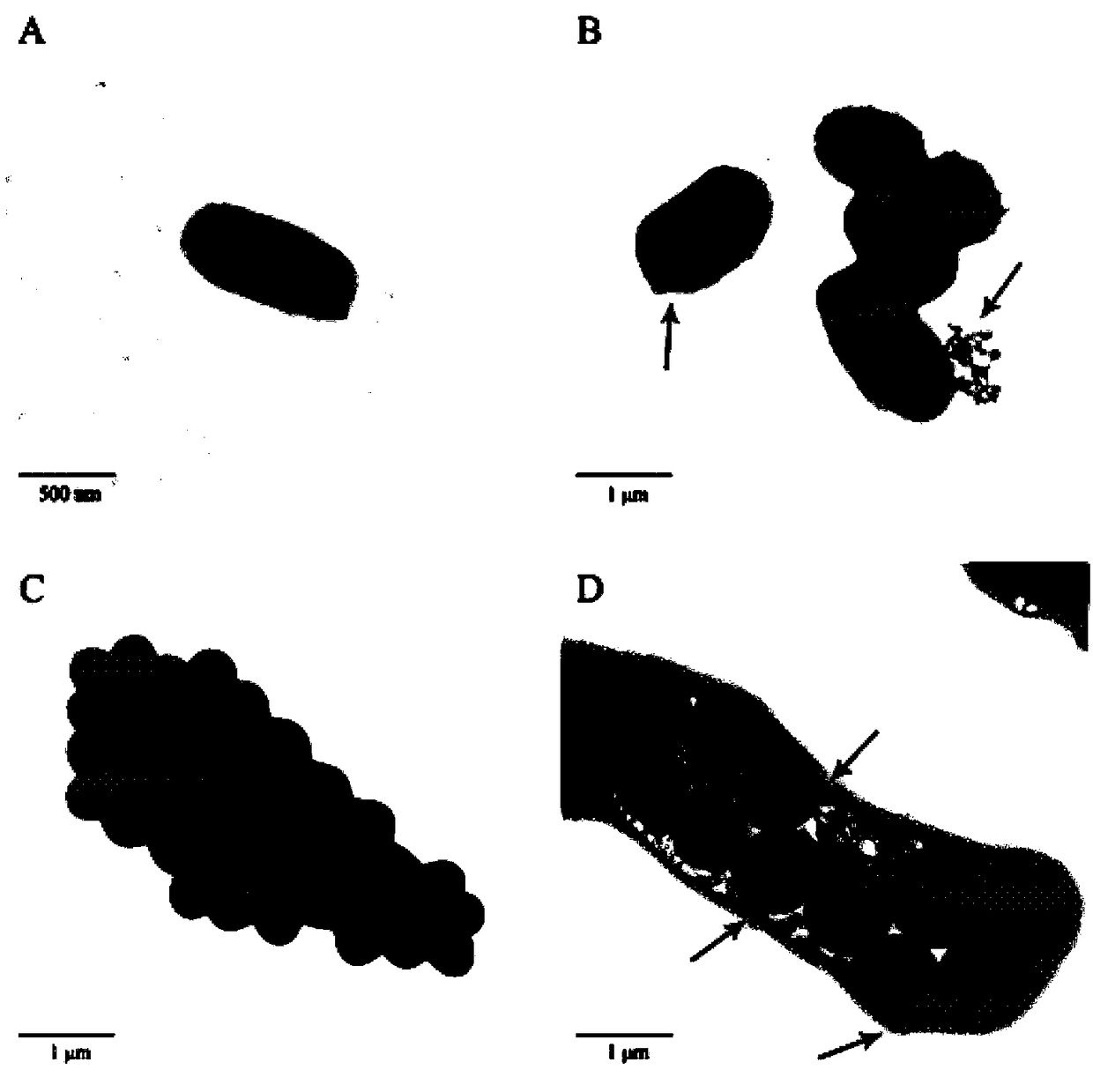
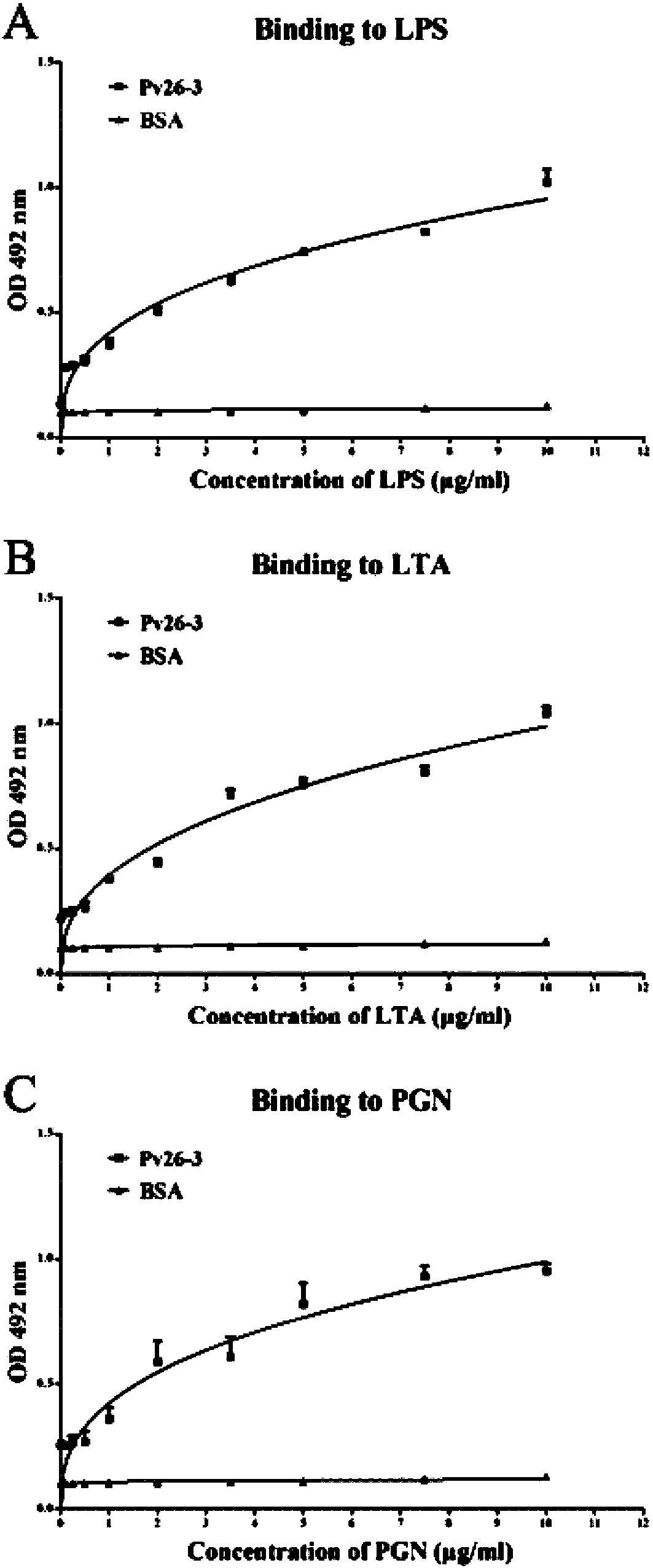
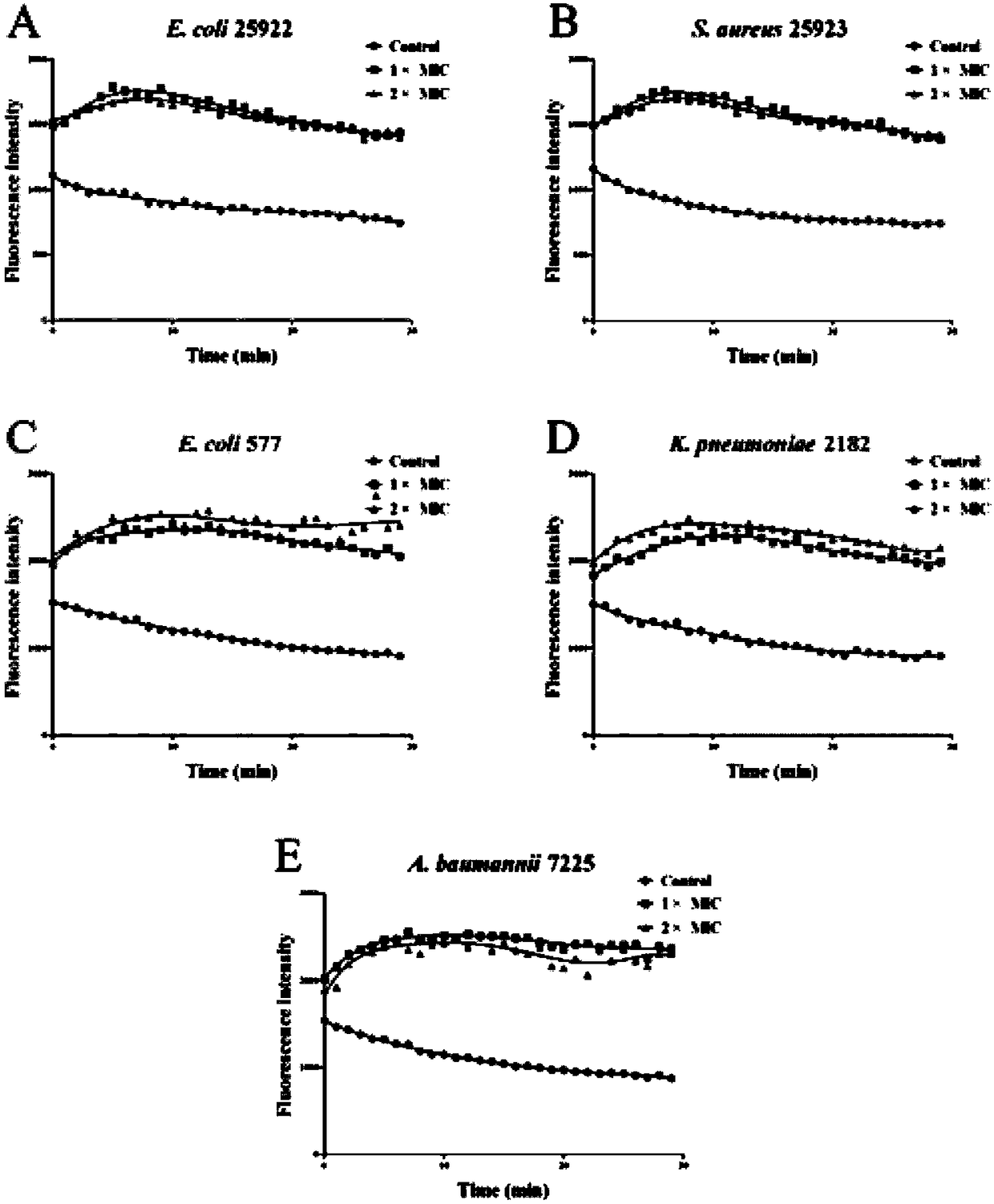
Polypeptide Pv26-3 with antimicrobial activity
An antibacterial activity, pv26 technology, applied to the polypeptide Pv26-3. It can solve the problems of the endless emergence of pathogenic bacteria and the abuse of antibiotics, and achieve the effect of enhancing the binding ability, reducing the concentration of action, and improving the antibacterial effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] In order to make the objectives, technical solutions, and advantages of the present application clearer, the implementation manners of the present application will be described in further detail below.
[0021] In the following, a polypeptide Pv26-3 with antibacterial activity according to an embodiment of the present invention will be described in detail.
[0022] The polypeptide Pv26-3 with antibacterial activity in the embodiment of the present invention is a mutant of the parent molecule Pv26 obtained by truncation of the phosphoprotein sequence, wherein the amino acid sequence of the parent molecule Pv26 is DTSSG SAAAS FEQMQ KQNRFLGNDIP, The amino acid sequence of the polypeptide Pv26-3 is KTSSG SWAAS WKQMQ KQNRK WGNKWP.
[0023] Specifically, the phosphoprotein used in the embodiment of the present invention is yolk phosphoprotein, which is a protein derived from degradation of yolk protein. The function of vitellin has always been considered to provide nutrients during...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com