Polycyclic aromatic hydrocarbon compound used as electroluminescent material and light-emitting device thereof
An electroluminescent material and aromatic ring technology, which can be applied to luminescent materials, compounds of Group 5/15 elements of the periodic table, compounds containing elements of Group 3/13 of the periodic table, etc., which can solve the problem of insufficient redox stability. , the emission spectrum is large, the color purity is not good enough, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
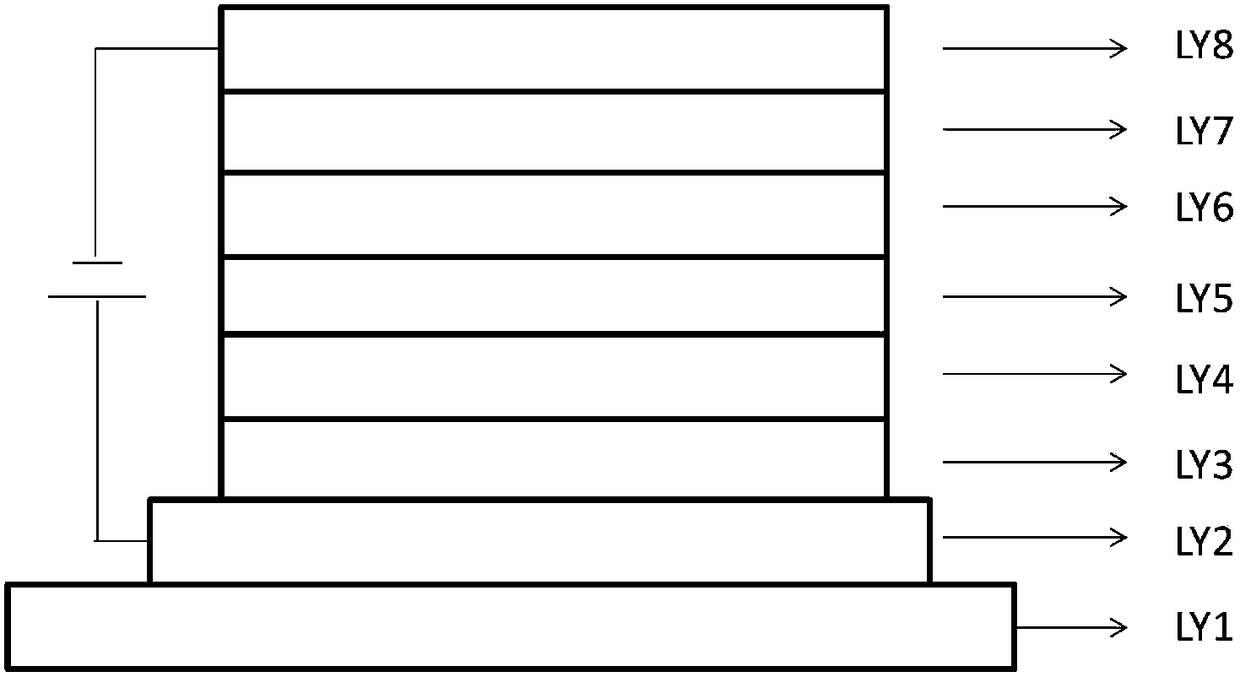
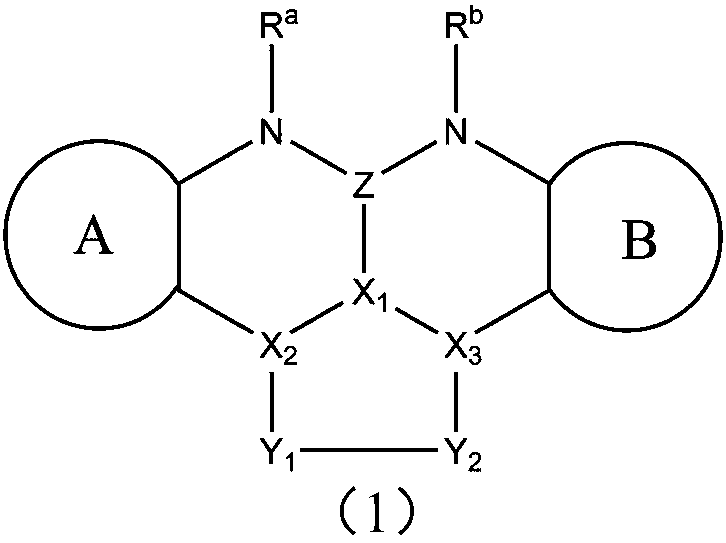
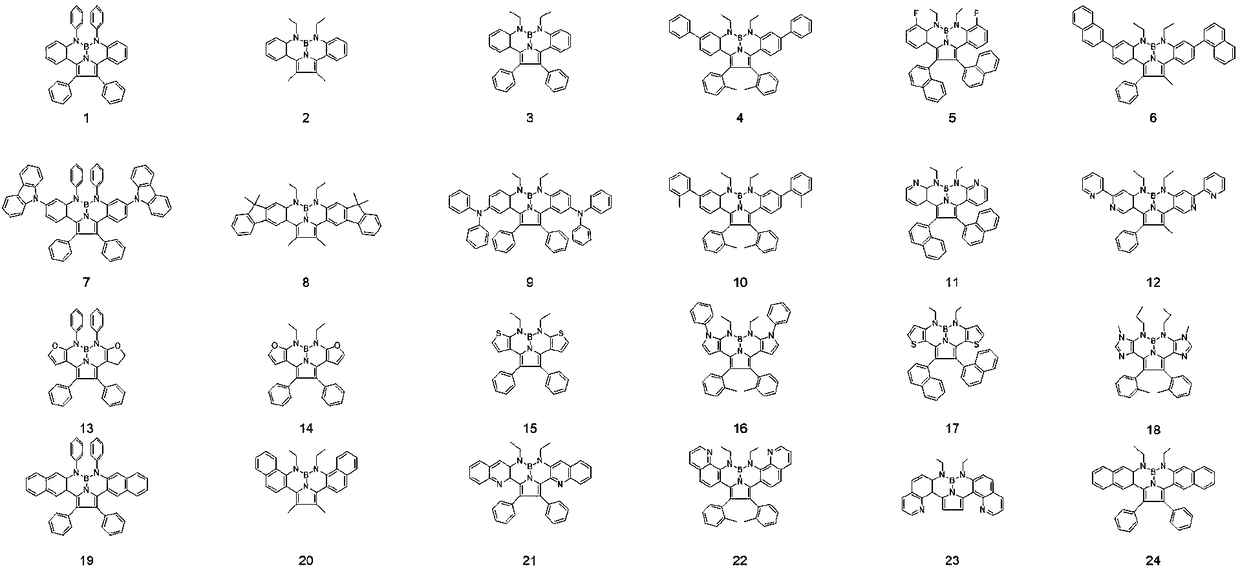
Image
Examples
Embodiment 1
[0047] For the synthesis of compound 1, the reaction equation is as follows:
[0048] (1)
[0049]
[0050] (2)
[0051]
[0052] Specific steps are as follows:
[0053] (1) In a 250mL flask, add 2-bromo-N-ethylaniline R A1 6.0g (30mmol), R C1 4.16g (12mmol), potassium carbonate 3.4g (25mmol), 100ml of toluene, 15ml of ethanol, 15ml of deionized water, under nitrogen protection, add tetrakistriphenylphosphine palladium Pd (PPh 3 ) 4 1.2g (0.1mmol), slowly heated to 65°C, stirred and refluxed for 18 hours, stopped the reaction, cooled to room temperature, separated liquids, collected the organic phase, and removed the solvent to obtain intermediate INT1 A1 2.9g (8mmol, yield 67%);
[0054] (2) Under the protection of nitrogen, the intermediate INT1 A1 Dissolve 2.9g (8mmol) in 100ml o-xylene, then add 2.9ml of 2.5mol / L n-butyllithium hexane solution dropwise. After the addition is complete, heat up to 70°C, stir for 4 hours, and then heat up to 100°C Then the hexane was distilled off. A...
Embodiment 2
[0056] Refer to the method of Example 1 to synthesize compound 3, the difference is that in step (2), R C2 Instead of R C1 , Compound 3 is obtained through the same reaction.
Embodiment 3
[0058] Compound 8 was synthesized by referring to the method in Example 1, except that in step (1), R A2 Instead of R A1 , Compound 8 was obtained through the same reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com