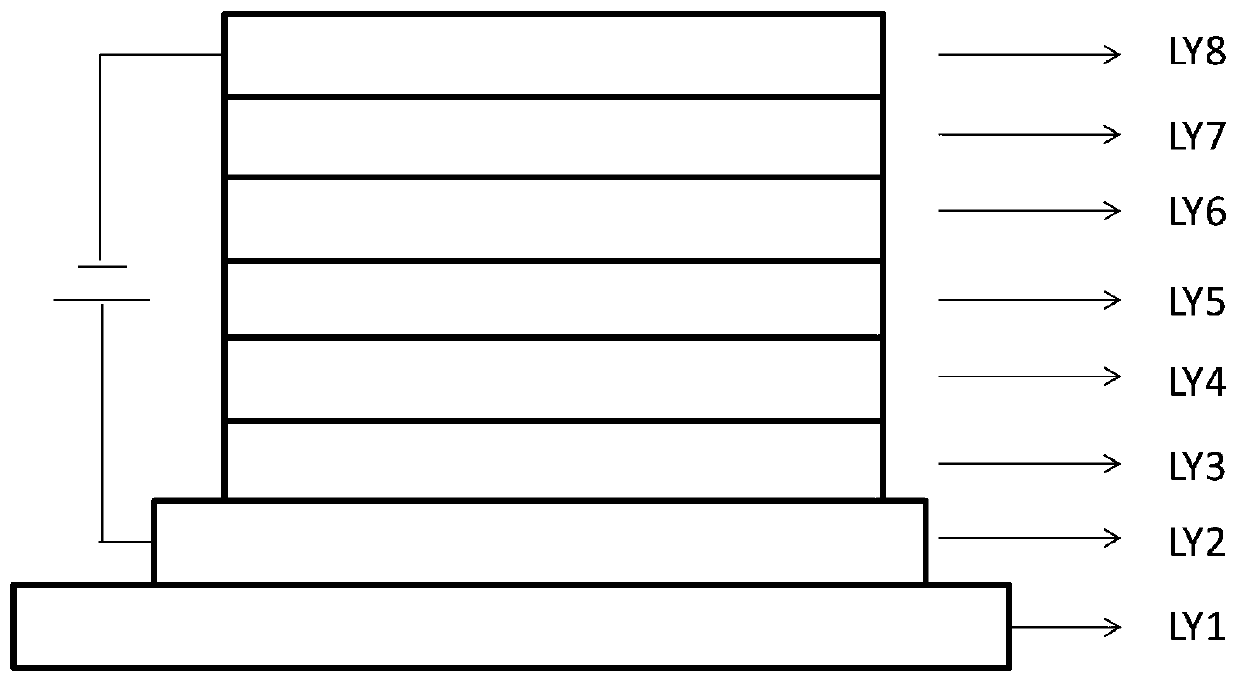
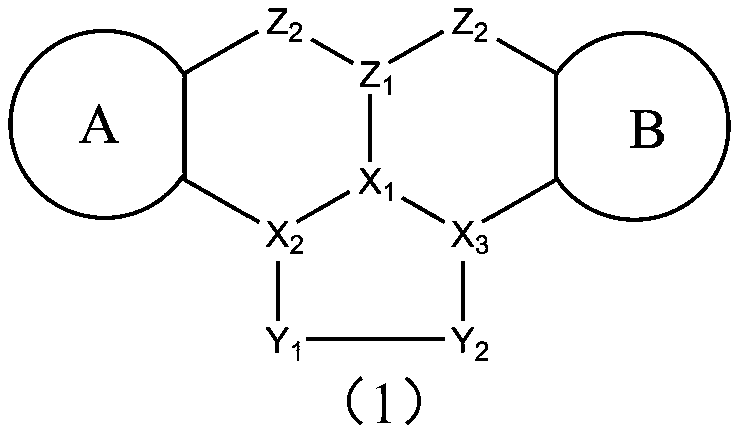
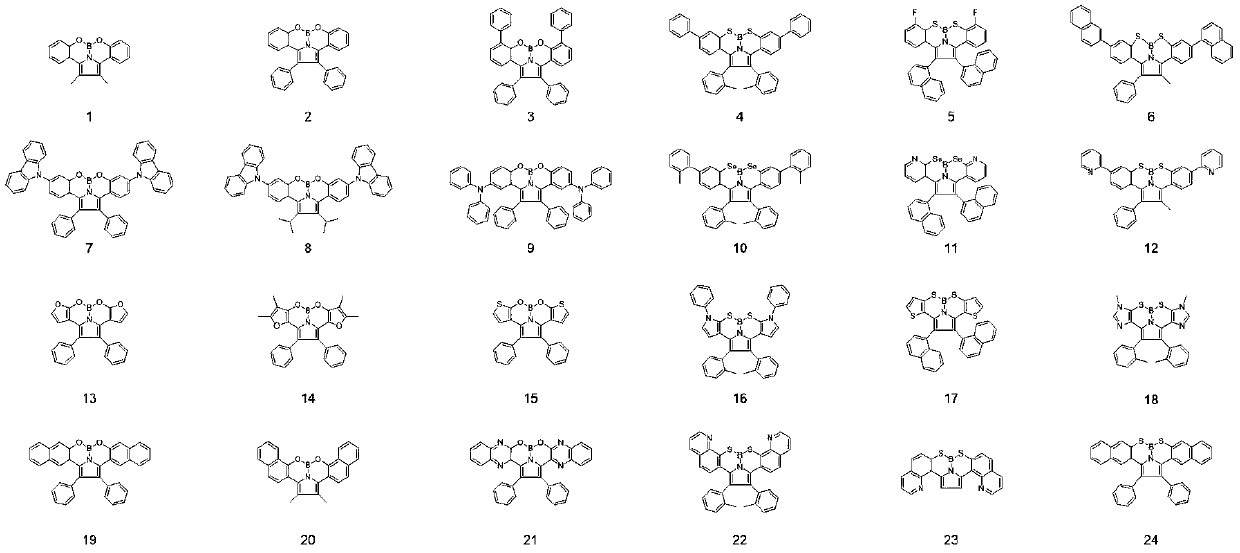
A class of polyaromatic ring compounds used as electroluminescent materials and light-emitting devices thereof
A technology of electroluminescent materials and aromatic rings, which is applied in the direction of luminescent materials, compounds of group 5/15 elements of the periodic table, compounds containing elements of group 3/13 of the periodic table, etc., and can solve the problem of insufficient oxidation-reduction stability , low-energy triplet excited state, not suitable for blue light-emitting materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of compound 1, the reaction equation is as follows:
[0049] (1)
[0050]
[0051] (2)
[0052]
[0053] (3)
[0054]
[0055] (4)
[0056]
[0057] The specific synthesis process is as follows:
[0058] (1) the o-hydroxybromobenzene R A1 (5.16g, 30mmol) was dissolved in 120ml of ethanol, and then added benzyl chloride (BnCl, 3.79g, 30mmol), potassium iodide (KI, 0.175g, 1mmol) and potassium carbonate (K 2 CO 3 , 2.48g, 18mmol), stirred, heated to 85°C, refluxed for 5 hours; cooled to room temperature, extracted 3 times with diethyl ether (15ml), combined organic layers, dried over anhydrous magnesium sulfate, concentrated, and the residue obtained pale yellow by column chromatography Liquid INT1 A1 7.07g;
[0059] (2) The INT1 obtained in the previous step A1 (7.07g), R C1 3.47g (10mmol), potassium carbonate 3.4g (25mmol), toluene 120ml, ethanol 15ml and deionized water 15ml join in the 250ml flask, add tetrakistriphenylphosphine pal...
Embodiment 2
[0063] Synthetic compound 2 with reference to the method for embodiment 1, difference is that adopt R in step (2) C2 instead of R C1 , Compound 2 was obtained through the same reaction.
Embodiment 3
[0065] Compound 13 was synthesized with reference to the method of Example 1, except that R was used in step (1). A2 Ligand R A1 , using R in step (2) C2 Ligand R C1 , Compound 13 was obtained through the same reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com