Tri-functionality PNIMMO energy-containing adhesive and synthesis method thereof
A technology of trifunctionality and synthesis method, applied in the direction of explosives, etc., can solve the problems of not easy to stir evenly, high viscosity of the system, etc., and achieve the effects of wide range of curing agents, reduced viscosity, and shortened curing time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
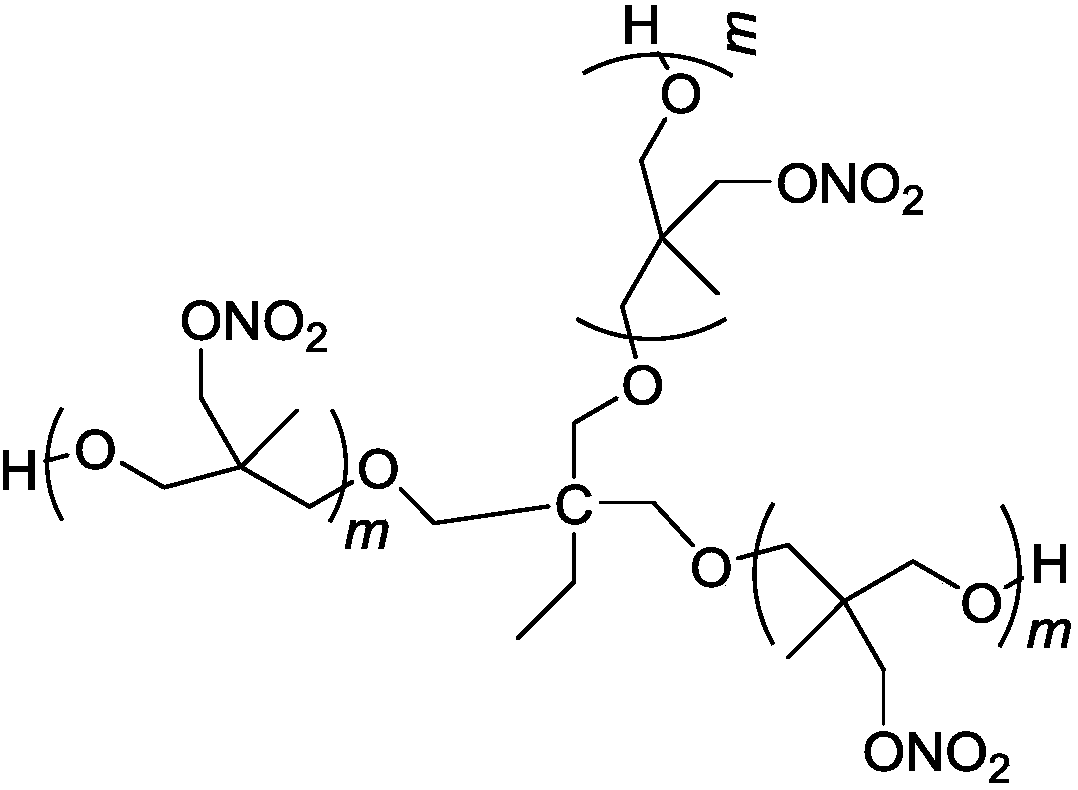
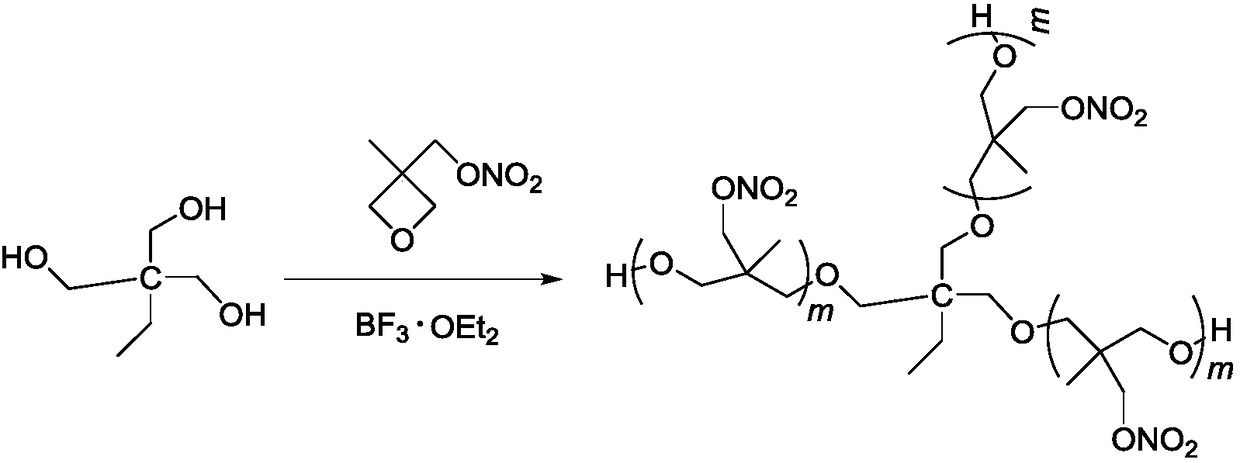
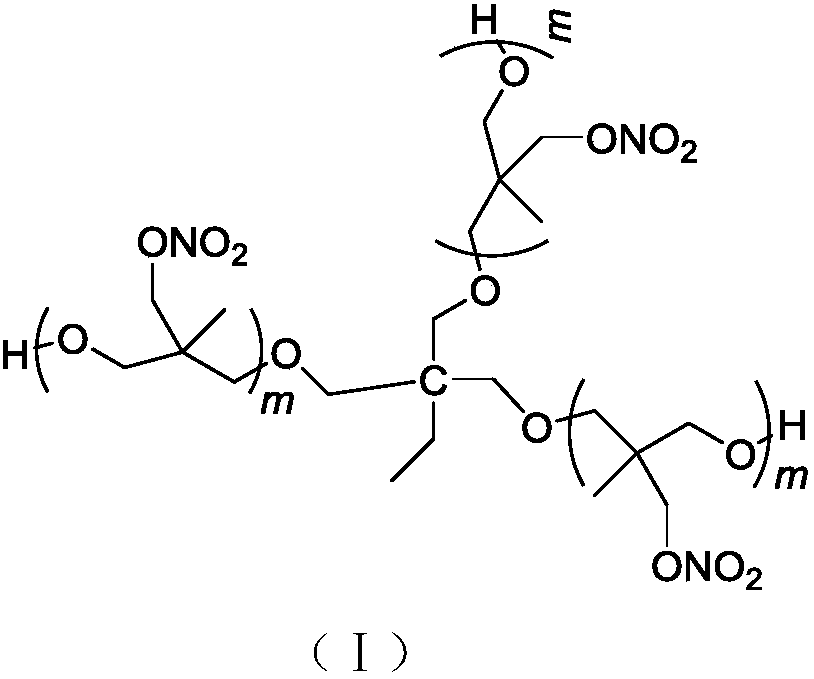
[0023] In a 100mL four-neck round bottom flask equipped with mechanical stirring, reflux condenser, thermometer, and dropping funnel, add 20mL of dichloromethane, 1.3g (10mmol) of trimethylolpropane (TMP) and 1.4g of trifluorinated For the boron ether complex, after stirring for 30 minutes at a temperature of 20°C to 30°C, 22g (150mmol) of 3-nitratemethyl-3-methyloxetane (NIMMO) was added dropwise, and the dropwise addition time For 2h, continue to react for 24h after the dropwise addition, use Na 2 CO 3 The aqueous solution was neutralized, the organic phase was washed with water until neutral, and the solvent was distilled off under reduced pressure to obtain 22 g of light yellow viscous liquid.
[0024] Structure Identification:
[0025] IR, ν max (cm -1 ): 3444 (-OH), 1112 (fatty ether C-O-C), 1630, 1281, 870 (-ONO 2 ).
[0026] 1 H NMR (CDCl 3 ,500MHz): δ5.31(s,1H),4.31~4.49(m,2H),3.25~3.37(m,4H),2.37(m,3H); 13 C NMR (CDCl 3 ,125MHz): δ75.84, 74.93, 73.77, 71.51...
Embodiment 2
[0030] In a 100mL four-neck round bottom flask equipped with mechanical stirring, reflux condenser, thermometer, and dropping funnel, add 20mL of dichloromethane, 1.3g (10mmol) of trimethylolpropane (TMP) and 1.4g of trifluorinated For the boron ether complex, after stirring for 30 minutes at a temperature of 20°C to 30°C, 33g (225mmol) of 3-nitratemethyl-3-methyloxetane (NIMMO) was added dropwise, and the dropwise addition time For 3h, continue to react for 24h after the dropwise addition, use Na 2 CO 3 The aqueous solution was neutralized, the organic phase was washed with water until neutral, and the solvent was distilled off under reduced pressure to obtain 33 g of light yellow viscous liquid.
Embodiment 3
[0032] In a 100mL four-neck round bottom flask equipped with mechanical stirring, reflux condenser, thermometer, and dropping funnel, add 20mL of dichloromethane, 1.3g (10mmol) of trimethylolpropane (TMP) and 1.4g of trifluorinated For the boron etherate complex, after stirring for 30 minutes at a temperature of 20°C to 30°C, 44g (300mmol) of 3-nitratemethyl-3-methyloxetane (NIMMO) was added dropwise, and the dropwise addition time For 4h, continue to react for 24h after the dropwise addition, use Na 2 CO 3 The aqueous solution was neutralized, the organic phase was washed with water until neutral, and the solvent was distilled off under reduced pressure to obtain 44 g of light yellow viscous liquid.
[0033] The application properties of the trifunctionality PNIMMO energetic adhesive of the present invention:
[0034] 1) The curing time of the available diisocyanate curing agent
[0035] The trifunctional PNIMMO energetic adhesive prepared by the present invention is respe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com