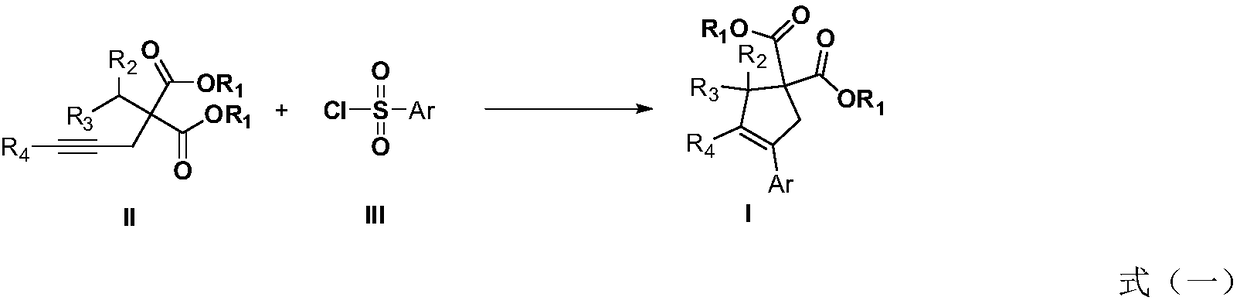
Method for performing C(sp3)-H functionalization cyclization reaction under photooxidation reduction catalytic system
A catalytic system, a technology of functional grouping, applied in chemical instruments and methods, organic chemistry, preparation of organic compounds, etc., can solve the problems of limited application and many organic side reactions, and achieve the effect of simple operation, low cost and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0037]
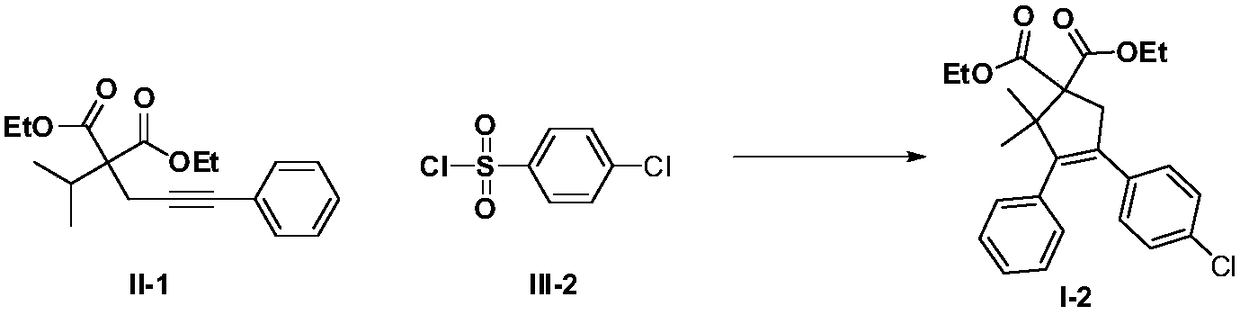
[0038] Using p-chlorobenzenesulfonyl chloride as the free radical donor, the rest of the operations were the same as in Example 1, and the yield of the target product I-2 was 76%.
Embodiment 12
[0040]
[0041] Using p-cyanobenzenesulfonyl chloride as the free radical donor, the rest of the operations were the same as in Example 1, and the yield of the target product I-3 was 84%.
Embodiment 13
[0043]
[0044] Using benzenesulfonyl chloride as the free radical donor, the remaining operations were the same as in Example 1, and the yield of the target product I-4 was 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com