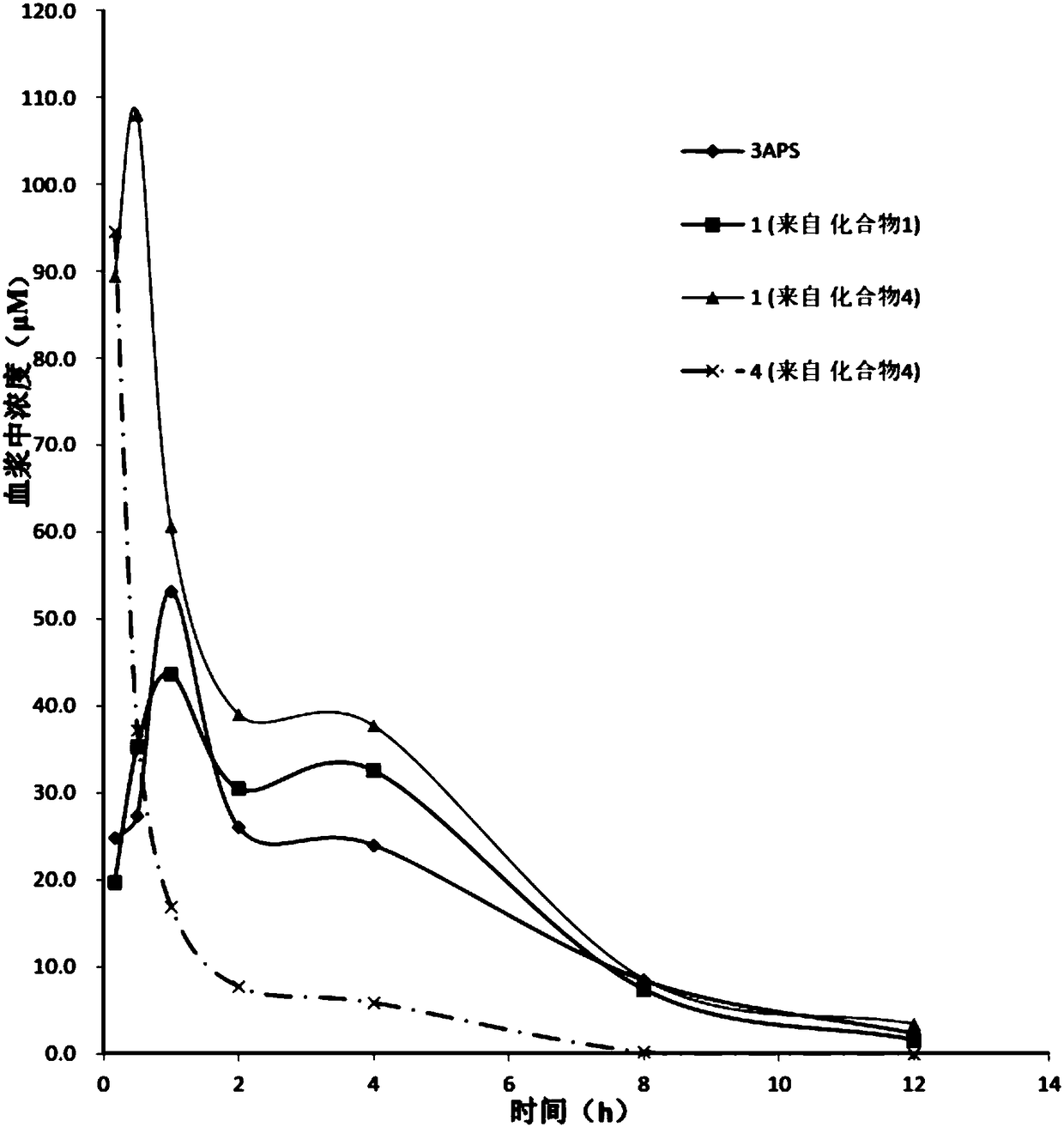
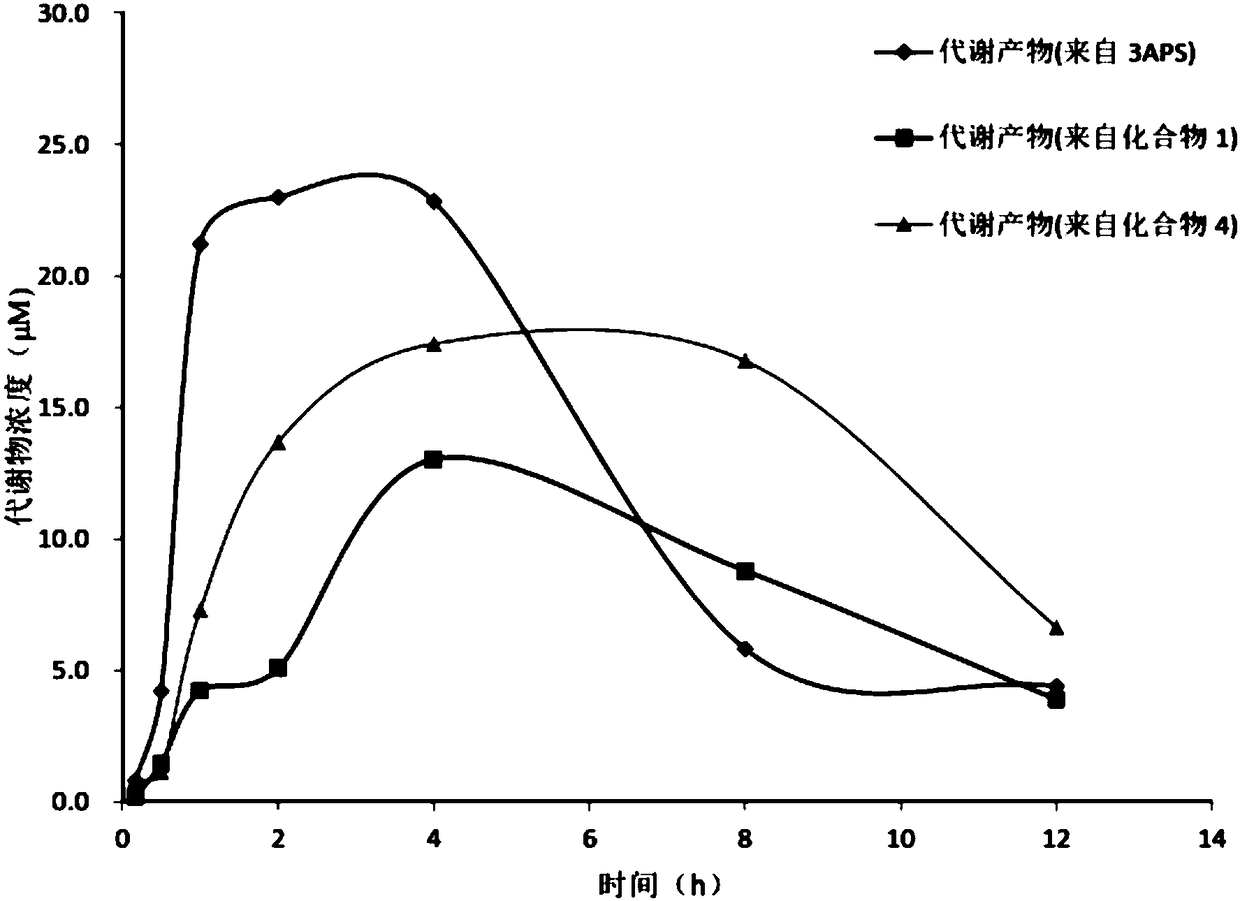
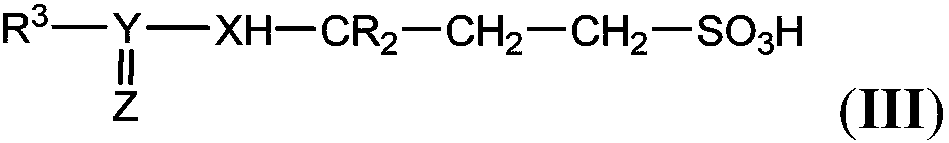Isotopically enriched 3-amino-1-propanesulfonic acid derivative and use thereof
An isotope enrichment and amino acid technology, applied in the direction of isotope introduction of heterocyclic compounds, isotope introduction of acyclic/carbocyclic compounds, medical preparations containing active ingredients, etc., can solve the problem of reduced reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] The preparation of the pharmaceutical composition can be carried out by methods known in the art (for example, referring to Remington: The Science and Practice of Pharmacy, 20 th Edition, 2000). For example, therapeutic compounds and / or compositions are combined with one or more pharmaceutical carrier substances and / or additives (or auxiliary substances) in solid or liquid form and, if desired, with other active compounds having a therapeutic or prophylactic effect, to form A suitable mode of administration or dosage form is then administered as a drug in humans or animals. Preparation of medicament can add many additives known in the art, such as fillers, disintegrants, binders, lubricants, wetting agents, stabilizers, emulsifiers, dispersants, preservatives, sweeteners, colorants, Flavoring agents, fragrances, thickeners, diluents, buffers, solvents, solubilizers, agents for depot effect, salts for changing osmotic pressure, coating agents or antioxidants, etc.
[...
Embodiment 1
[0169] Example 1: Synthesis of 3-amino-3,3-dideuterium-1-propanesulfonic acid (1) and 3-amino-3,3-dideuterium-1-propanesulfonic acid sodium salt (1s)
[0170] A solution of 3-hydroxypropionitrile (26.0g, 366mmol, 1.0eq.) in anhydrous THF (50mL) was slowly dropped into the solution containing LiAlD 4 (10.0 g, 238 mmol, 0.65 eq.) in anhydrous THF (200 mL), the reaction mixture was stirred under reflux overnight. After the reaction was cooled to room temperature, water (4.8 mL), 15% NaOH aqueous solution (4.8 mL) and water (14.4 mL) were added sequentially to quench, and stirred at room temperature for 2 hours, then filtered to remove solid impurities. The filtrate was concentrated under reduced pressure and dried to obtain a red oily liquid which was directly used in the next reaction without further purification.
[0171] The oily liquid (10.0g, 128mmol, 1.0eq.) was dissolved in 100mL of chloroform solution, stirred in an ice bath and slowly dropped into thionyl chloride (18.2...
Embodiment 2
[0174] Example 2: Synthesis of 3-((L-alanyl)amino)-3,3-dideuterium-1-propanesulfonic acid (2)
[0175]Compound 1s (0.30 g, 1.84 mmol, 1.0 eq.) and N-tert-butoxycarbonyl-L-alanine (0.37 g, 2.0 mmol, 1.1 eq.) were mixed in anhydrous DMF (10 mL). Add N,N'-dicyclohexylcarbodiimide (DCC, 0.56g, 2.7mmol, 1.5eq.) and 1-hydroxybenzotriazole (HOBt, 0.24g, 1.80mmol, 1.0eq. .) Stir overnight at room temperature. After adding water (2 mL), stirring was continued for one hour, the insoluble solid was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a crude white solid. The solid was dissolved in water (20 mL), extracted twice with ethyl acetate, the aqueous phase was concentrated under reduced pressure, and the residue was subjected to column chromatography to obtain a white solid 3-((N-Boc-L-alanyl)amino 0.50 g of sodium )-3,3-dideuterium-1-propanesulfonate, and the yield was 81.3%. The white solid (0.50 g, 1.50 mmol, 1.0 eq.) was dissolved in 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com