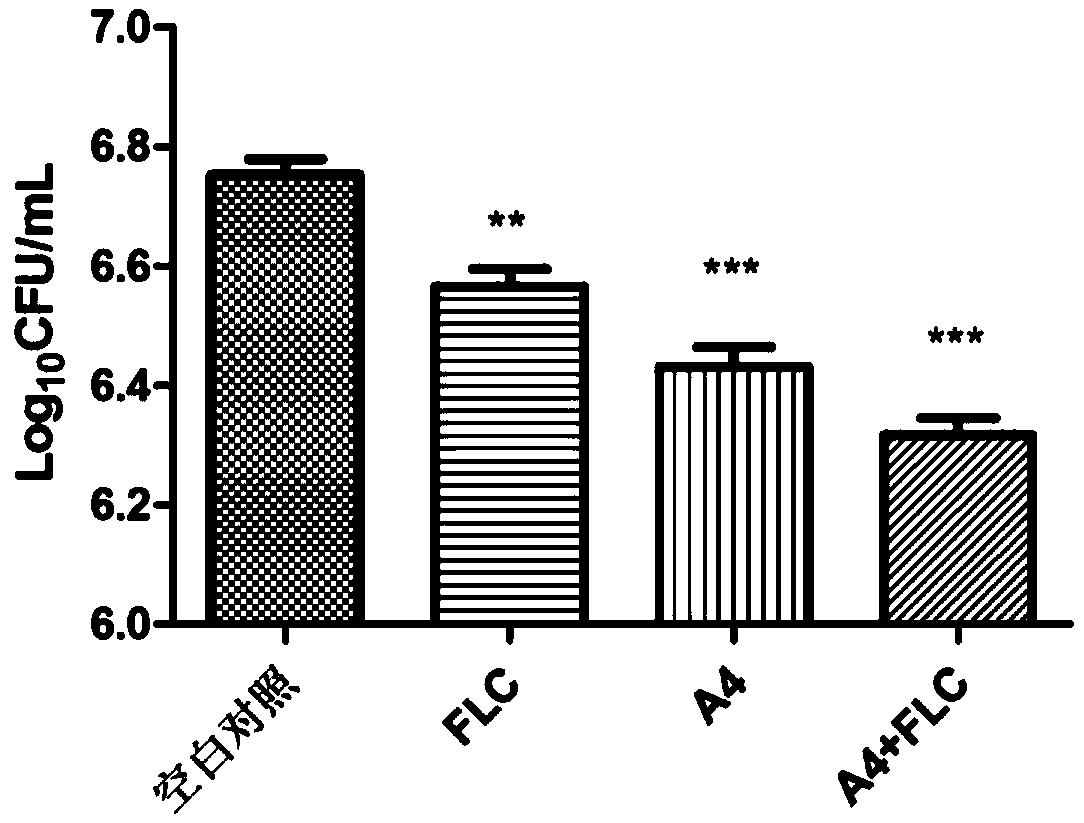
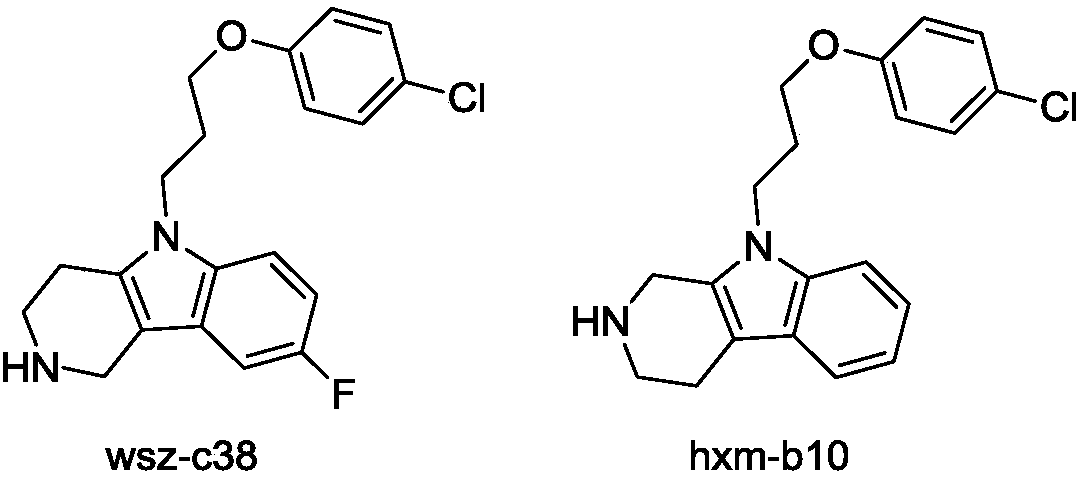
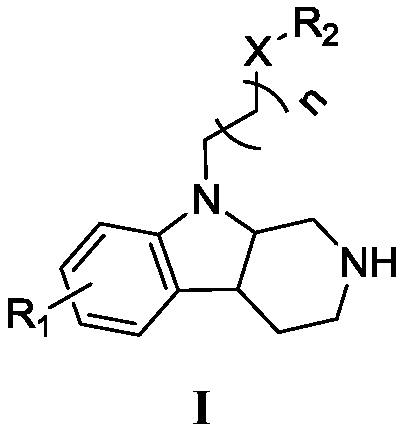
Beta- tetrahydro-carboline antifungal drug and preparation method and application thereof
A tetrahydrocarboline, antifungal technology, applied in the field of medicine, can solve the problems of no antifungal effect, limited bacteriostatic effect, unclear structure-activity relationship and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0078] 1. Preparation of compound A series
[0079] Synthetic route 1
[0080]
[0081] Reagent conditions: (a) POCl 3 , DMF, rt, yield 81-92%; (b) NH 4 OAc,CH 3 NO 2 , rf, yield 78-91%; (c) LiAlH 4 , THF, rf, yield 37-78%; (d) AcOH:MeOH=10:1, yield 45-90%; (e) THF, rt, 1h, yield 51-89%; (f) K 2 CO 3 , EtOH, yield 51-66%; (g) NaH, DMF, yield 34-54%; (h) TFA, CH 2 Cl 2 , yield 71-85%.
[0082] In the above reagent conditions, "rt" means normal temperature reaction, "yield" means yield, and "rf" means reflux, the same below.
[0083] The preparation process of compound A1-A29 is as follows:
[0084] (a1) Preparation of compound 2-6
[0085] Indole and POCl 3 React in DMF solvent at room temperature to obtain compound 2. Compound 2 was dissolved in nitromethane, and compound 3 was catalyzed by ammonium acetate. Compound 3 was dissolved in anhydrous tetrahydrofuran as a solvent, and lithium aluminum tetrahydrogen was added to react under anhydrous conditions to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com