Flexible aromatic diamine monomer containing aromatic ring side group and preparation method thereof
A technology of aromatic diamines and side groups, which is applied in the field of flexible aromatic diamine monomers and its preparation, can solve the problems of main chain flexibility and aromatic ring side groups, and achieve the effect of improved processability and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
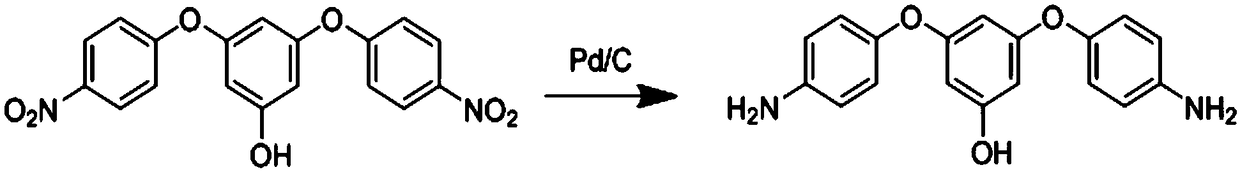
[0027] Specific implementation mode 1: A flexible aromatic diamine monomer containing aromatic ring side groups according to this implementation mode, its chemical formula is C 31 h 24 N 2 o 4 , the structural formula is:
[0028]
specific Embodiment approach 2
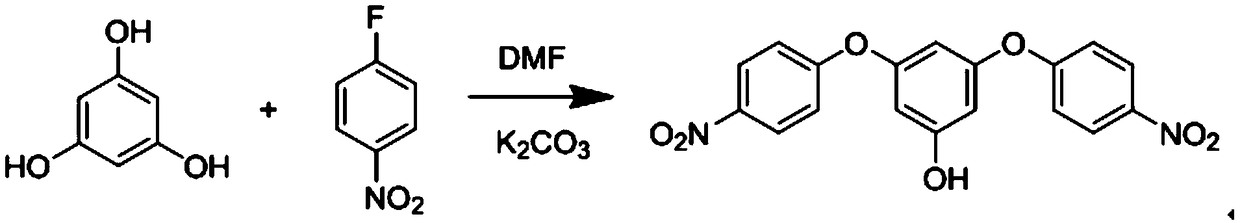
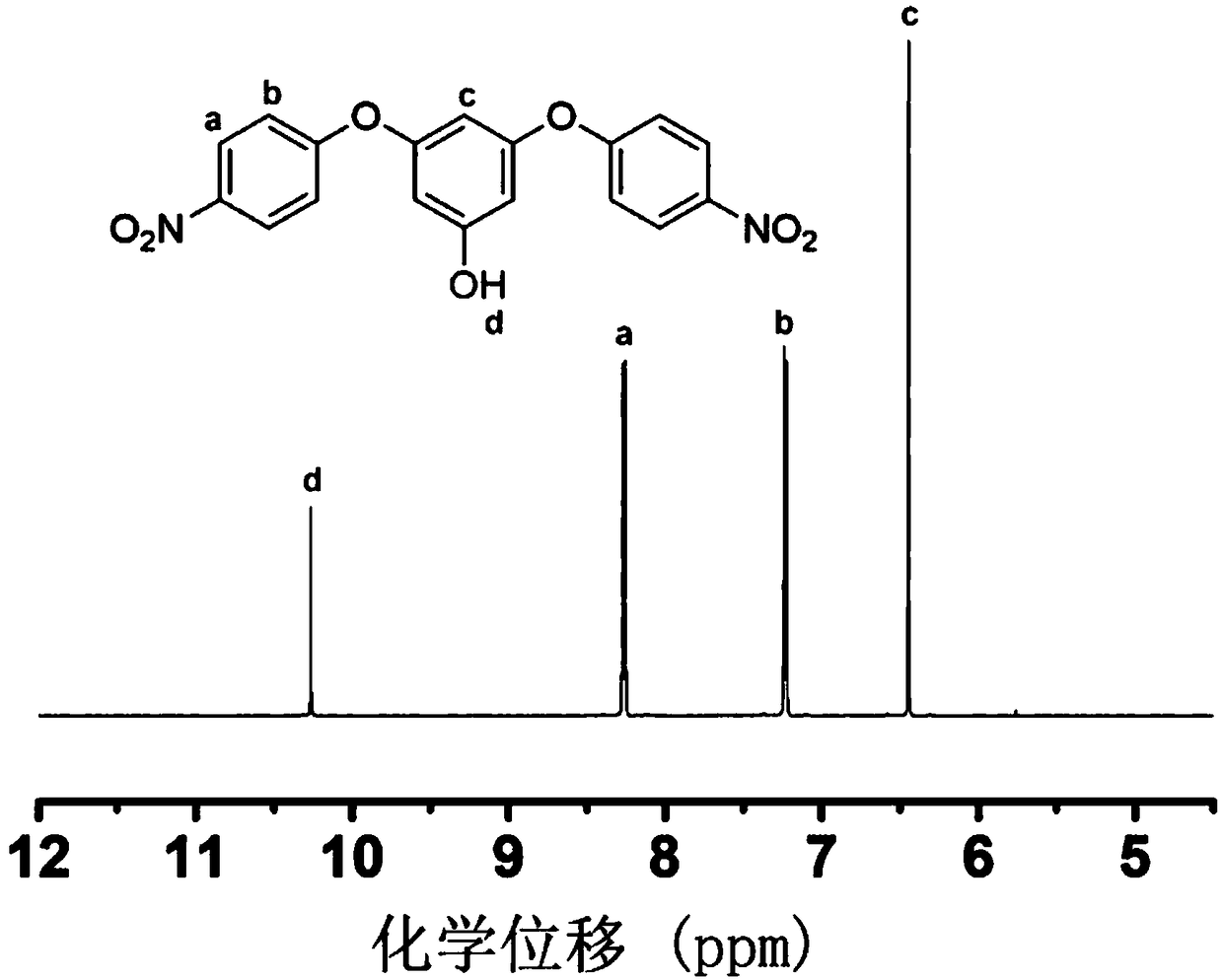
[0029] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the initial raw materials for preparing flexible aromatic diamine monomers containing aromatic ring side groups are phloroglucinol and p-fluoronitrobenzene; The monomer obtained by reaction in N-dimethylformamide. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0030] Specific embodiment three: the difference between this embodiment and specific embodiment one is: the reaction product of the first step in the reaction is 3,5-bis(4-nitrophenoxy)phenol, chemical formula: C1 8 h 12 N 2 o 7 ;Structural formula:
[0031]
[0032] Others are the same as in the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com