Preparation method for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
A halogenated acetic acid and compound technology, applied in the field of medicinal chemistry, can solve the problems of unknown impurities, cumbersome process, low production capacity of intermediate DOTA, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059]
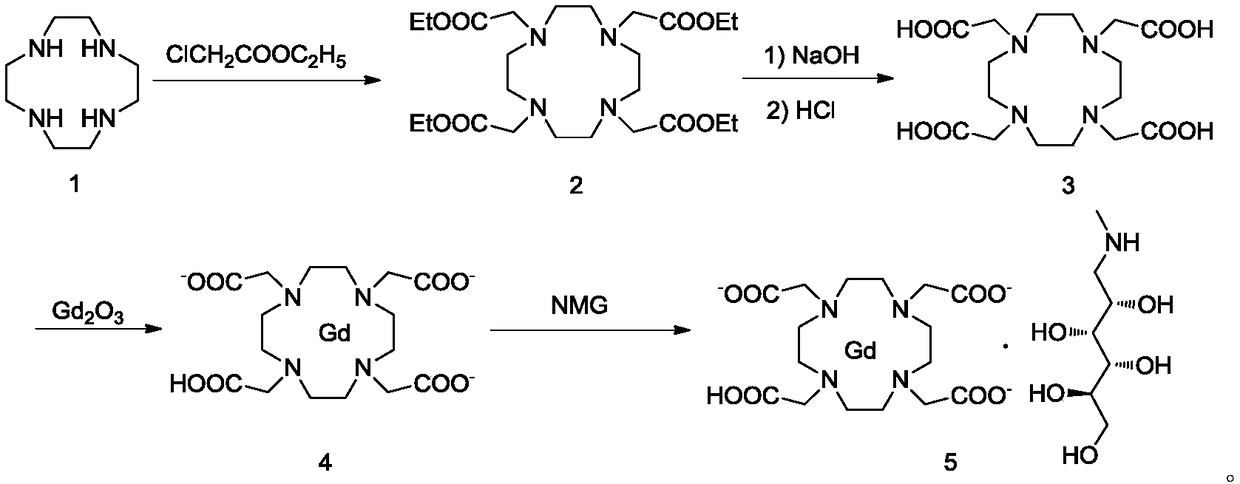
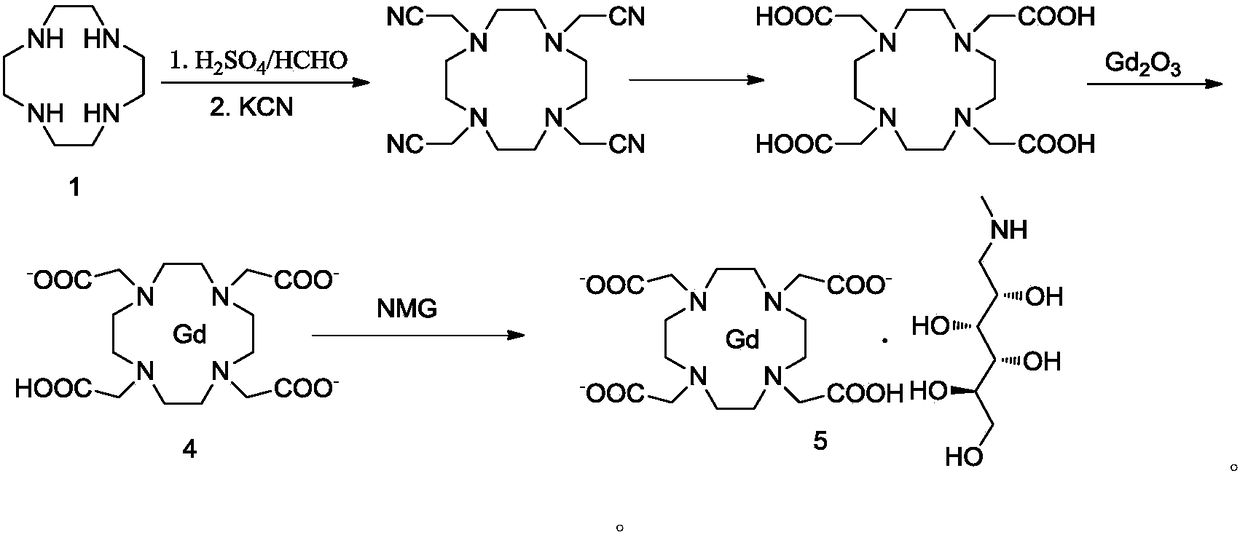
[0060] Add 11.8kg of chloroacetic acid and 12l of purified water into a 100L reactor, stir and dissolve, add 15l of water dissolved in 5kg of sodium hydroxide under an ice bath, suspend 3.2kg of rhodotendine in 6l of water and add it to the reaction, and heat up to 80~85℃, add dropwise 6L water dissolved with 5.2kg sodium hydroxide, control the pH of the reaction system at 10~11, react overnight, after cooling to room temperature, add 10.4l concentrated hydrochloric acid dropwise, adjust the pH to 2~2.5, stir and analyze crystal, the filter cake was collected by filtration, and dried to obtain 7.5 kg of DOTA crude product, the content of the compound of formula II was about 3.1%.
[0061] Add 7.5kg of crude DOTA and 18kg of purified water into a 50L reactor, heat and stir to dissolve, then cool and crystallize, filter, wash, and dry to obtain 6.21kg of DOTA with a yield of 82.7%, and the salt content is 2%, the compound of formula II The content is 0.05%.
Embodiment 2
[0062] Example 2: Exploration of DOTA Crude Refining Method
[0063] Take 20g DOTA crude product and put it into the reaction bottle, add the solvent to be screened respectively, after heating and dissolving, cool and stir to crystallize, filter to obtain DOTA samples, detect them respectively, and record the data as shown in Table 1 below:
[0064] Table 1
[0065]
[0066] Note:
[0067] a In the solvent screening, it can be detected by TLC (plate layer chromatography), and after obtaining a general evaluation, it can be further detected by HPLC and other detection means;
[0068] b After HPLC detection, the obtained DOTA was purified to 96.9%, with a salt content of 3.07%, and a compound of formula II of 0.02%;
[0069] c is detected by HPLC, and the obtained DOTA is purified to 95.7%, with a salt content of 10.27% (measured by titration method), and the compound amount of formula II is 0.05%;
[0070] d is detected by HPLC, and the obtained DOTA is purified to 95.1%,...
Embodiment 3
[0075]
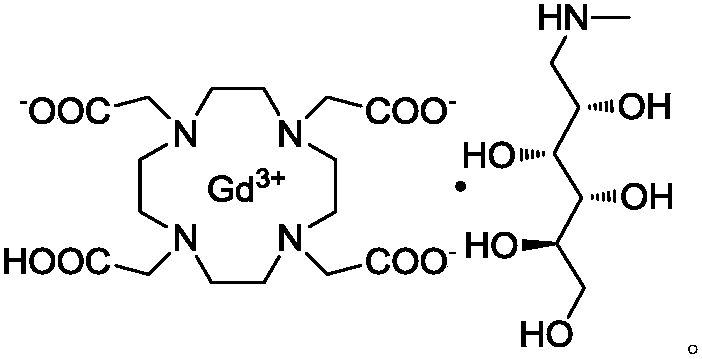
[0076] In 50L of purified water, add 5.1kg of DOTA obtained in Example 1, heat and stir at 40-50°C to dissolve, add meglumine (2.5kg) and gadolinium oxide (2.33kg) respectively, the solution pH=7-9, and heat to react 3h, filtered, desalted with 001×7 and D311 resin in turn to obtain gadoterate meglumine solution that meets the requirements, concentrated to dryness or lyophilized to obtain 7.46kg of gadoterate meglumine, yield 78.5%, tested The formula II content is less than 0.01%, the free gadolinium content is less than 0.013%, and the salt content is zero.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com