Medical application of brain and heart beneficial tablets in treatment of Alzheimer's disease
A technology for Alzheimer's disease and heart tablets, applied in the field of medicine, can solve the problems of "Linaoxin tablets" that have not been reported in relevant literature for the prevention and treatment of Alzheimer's disease, and achieve the prevention and treatment of Alzheimer's disease. Symptoms and diseases, the effect of obvious medical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] experimental method:
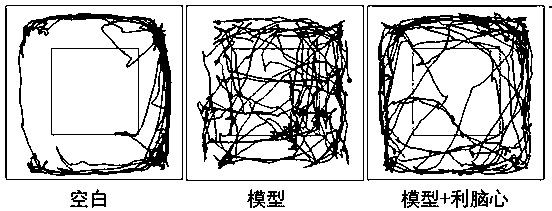
[0019] Set up a soundproof obscura, the bottom area is 30 cm x 30 cm, the central area is 15 cm x 15 cm, and the rest is used as the surrounding area. On the day of testing, the mice were placed in a fixed position in the box, and their movement trajectories and the time spent exploring the central area within 5 min were observed and recorded.
[0020] Experimental results:
[0021] The mice in the blank group preferred the surrounding areas and rarely entered the central area. Compared with the blank group mice, the mice in the model group had chaotic trajectories, and the time in the central area increased significantly, while the time in the four-week area decreased significantly. After 4 weeks of administration of Linaoxin, the exercise time of the mice in the four-week area was significantly longer than that of the model group (see figure 1 ).
Embodiment 2
[0023] experimental method:
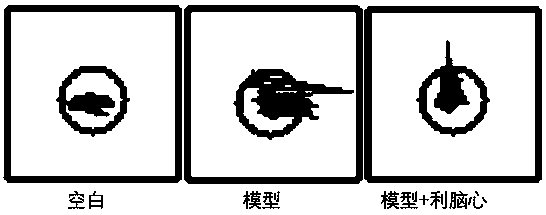
[0024] Training period: During training, the mice were put into the platform box to adapt to it for 5 minutes, and an alternating current was passed through the electric rod at the bottom, and the animal received an electric shock. The normal reaction was to jump on the platform to avoid the noxious stimulus. If the animal did not jump off the platform during the training period, it was discarded.
[0025] Test period: 24 hours after the end of the training period, put the mouse on the platform again, observe for 5 minutes, and record the time required for the animal to jump off the platform for the first time, which is the platform jumping latency.
[0026] Experimental results:
[0027] During the training period, the mice in each group would jump off the platform within 5 minutes. During the test on the second day, most of the mice in the blank group stayed on the platform within 5 minutes, while most of the mice in the model group jumped off ...
Embodiment 3
[0029] experimental method:
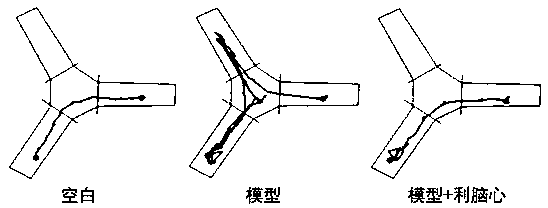
[0030] Training period: Put a little food on the edge of the C wall of the Y maze. After 12 hours of food deprivation, the mice are put into the Y maze from the A wall, adapt to it for 5 minutes, observe how the mice find the food, and conduct a formal test after 3 days of training. .
[0031] Test period: still put a little food on the edge of the C wall of the Y maze where the mice were deprived of food. After 12 hours of deprivation, the mice were put into the Y maze from the A wall, and the time when the mice found the food for the first time was observed.
[0032] Experimental results:
[0033] During the training period, there was no significant difference in the time for the mice in each group to find food. After 3 days of training, the time for the mice in the blank group to find food was gradually shortened, while the time for the mice in the model group to find food was significantly longer than that in the blank group. Compared with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com