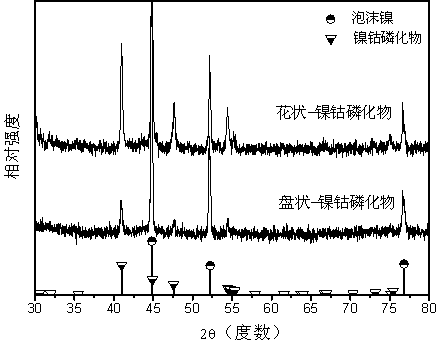
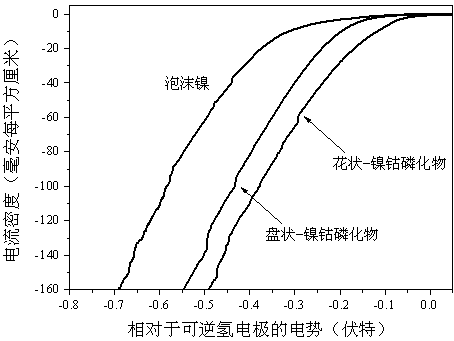
Preparation method of self-assembled ultrathin flower-like nickel cobalt phosphide electrocatalytic material
A catalytic material and ultra-thin flower technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problem of harsh preparation conditions of transition metal phosphide, unsatisfactory carrier dispersion effect, and cumbersome precipitant. Addition and other issues, to achieve the effect of low cost, increase contact area, and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation process of a self-assembled ultra-thin flower-shaped nickel-cobalt phosphide material is as follows:
[0046] (1) To remove the oxide layer on the surface of nickel foam, first soak it in acetone for 15 minutes, then soak it in 2M dilute hydrochloric acid solution for 15 minutes, wash it with a large amount of deionized water, and dry it in vacuum;
[0047] (2) Dissolve 0.17g nickel nitrate hexahydrate and 0.34g cobalt nitrate hexahydrate in 35mL deionized water and ethanol solution (V 水 :V 乙醇 =4:3), stir well until a red transparent solution is formed;
[0048] (3) Then add 0.2g of surfactant cetyltrimethylammonium bromide to the above solution, stir to make it fully dissolve to form a red solution;
[0049] (4) Pour the solution obtained in step (3) into the inner liner of a 50mL reactor, place a piece of processed nickel foam (2×4cm 2 ), incubate at 140°C for 10h and then naturally cool to room temperature to obtain the precursor;
[0050] (5) Cro...
Embodiment 2
[0054] (1) To remove the oxide layer on the surface of nickel foam, first soak it in acetone for 15 minutes, then soak it in 2M dilute hydrochloric acid solution for 15 minutes, wash it with a large amount of deionized water, and dry it in vacuum;
[0055] (2) Dissolve 0.17g nickel nitrate hexahydrate and 0.34g cobalt nitrate hexahydrate in 35mL deionized water and ethanol solution (V 水 :V 乙醇 =4:3), stir well until a red transparent solution is formed;
[0056] (3) Then add 0.2g of surfactant cetyltrimethylammonium bromide to the above solution, stir to make it fully dissolve to form a red solution;
[0057] (4) Pour the solution obtained in step (3) into the inner liner of a 50mL reactor, place a piece of processed nickel foam (2×4cm 2 ), incubate at 100°C for 10h and then naturally cool to room temperature to obtain the precursor;
[0058] (5) Cross-wash the precursor obtained in step (4) with deionized water and absolute ethanol for 3 to 5 times, and dry the washed produ...
Embodiment 3
[0062] (1) To remove the oxide layer on the surface of nickel foam, first soak it in acetone for 15 minutes, then soak it in 2M dilute hydrochloric acid solution for 15 minutes, wash it with a large amount of deionized water, and dry it in vacuum;
[0063] (2) Dissolve 0.17g nickel nitrate hexahydrate and 0.34g cobalt nitrate hexahydrate in 35mL deionized water and ethanol solution (V 水 :V 乙醇 =4:3), stir well until a red transparent solution is formed;
[0064] (3) Then add 0.2g of surfactant cetyltrimethylammonium bromide to the above solution, stir to make it fully dissolve to form a red solution;
[0065] (4) Pour the solution obtained in step (3) into the inner liner of a 50mL reactor, place a piece of processed nickel foam (2×4cm 2 ), incubate at 200°C for 10h and then naturally cool to room temperature to obtain the precursor;
[0066] (5) Cross-wash the precursor obtained in step (4) with deionized water and absolute ethanol for 3 to 5 times, and dry the washed produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com