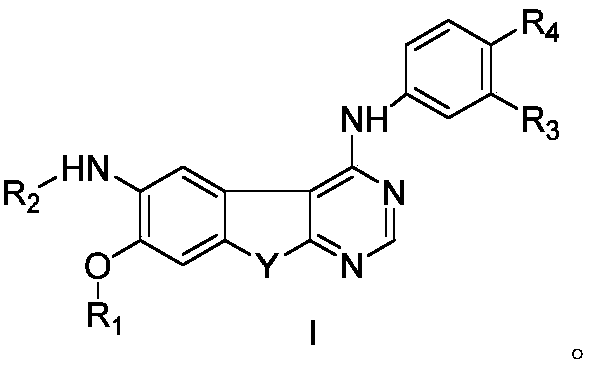
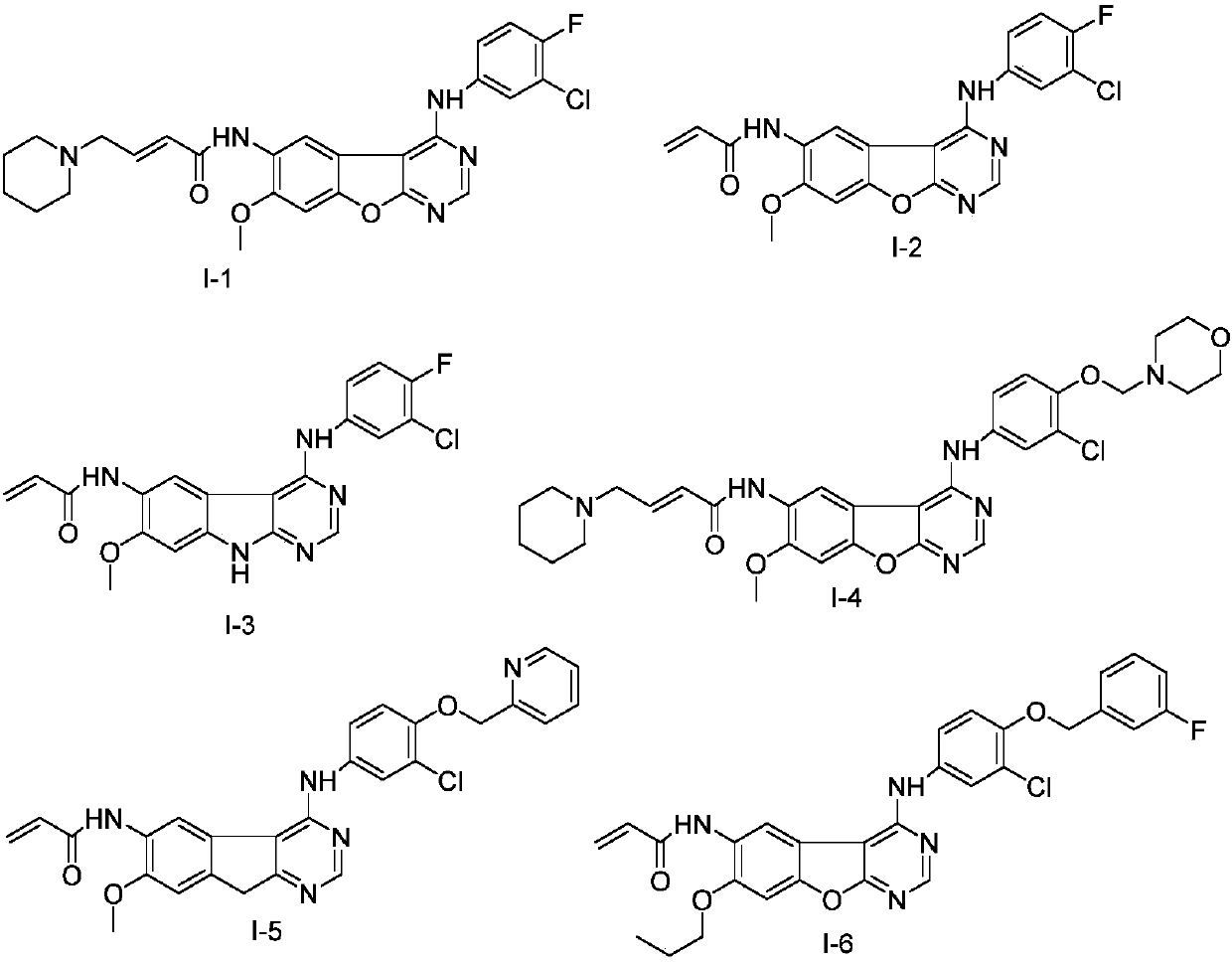
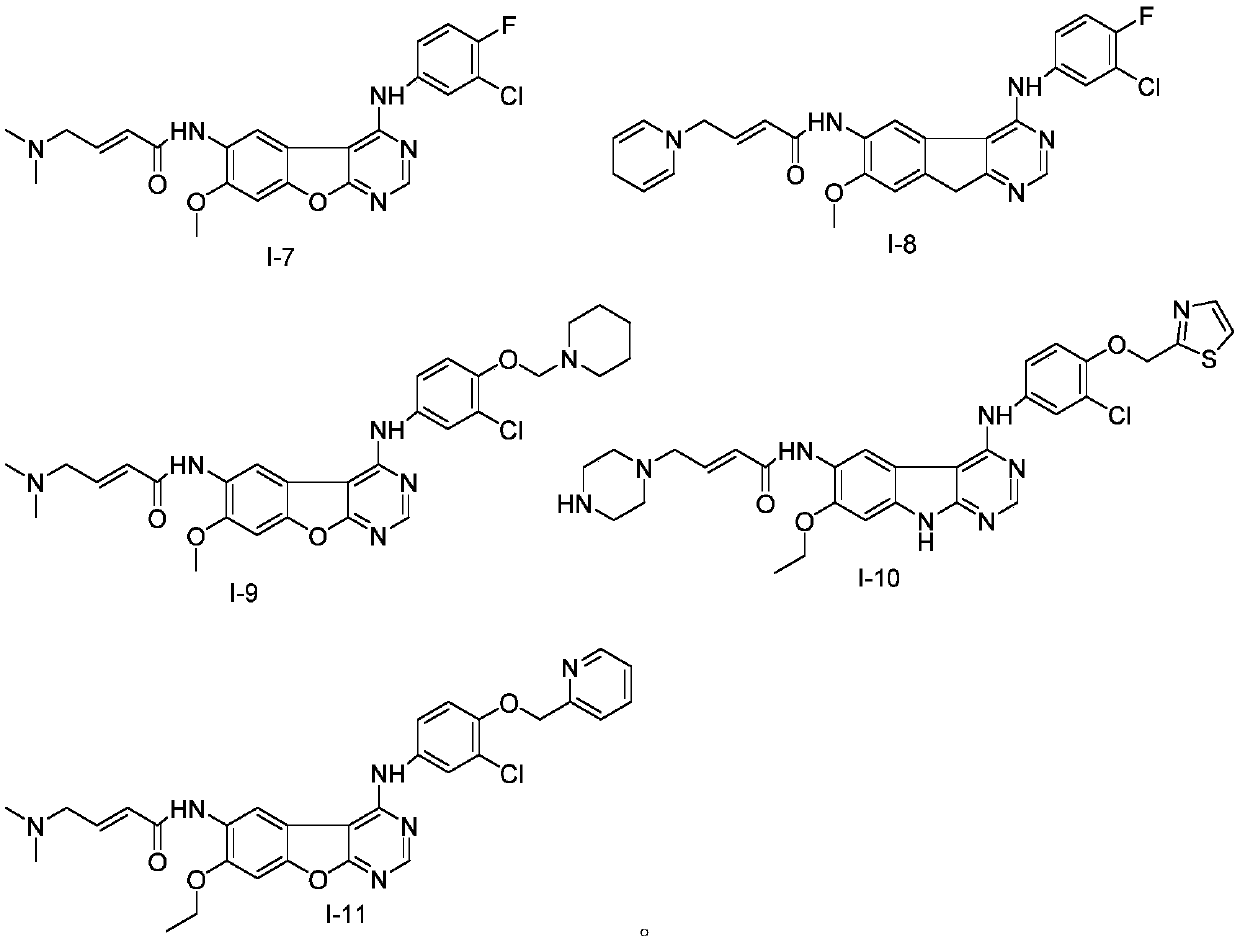
Pyrimidine derivative serving as HER2 tyrosine kinase inhibitor and application of pyrimidine derivative
A technology of tyrosine kinase, CH2, applied in the field of pyrimidine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: the preparation of the compound shown in formula I-1
[0088]
[0089] Under the condition of -5°C to 0°C, 19.1 g (0.102 moles) of the compound shown in formula a-1 was added to the reaction flask, and 35.8 g (0.1 moles) of the compound shown in formula g-1 and 160 A solution consisting of one milliliter of N-methylpyrrolidone was added within 20 minutes and reacted at room temperature for 12 hours. After TLC detected that the reaction was complete, an appropriate amount of water was added, and the resulting mixed solution was adjusted to pH 10.0 to 11.0 with 2 mol / liter aqueous sodium hydroxide solution, and a large amount of solid was precipitated, filtered by suction, washed, and dried to obtain a crude off-white solid. Recrystallized from methanol / acetone (methanol / acetone=3:1 (v / v)), purified to obtain a white solid product, 22.2 g of the compound shown in formula I-1, with a yield of 45.6% and a purity of 99.2%.
[0090] LCMS:511(M+1) + .
Embodiment 2
[0091] Embodiment 2: the preparation of the compound shown in formula I-2
[0092]
[0093] Under the condition of -5°C to 0°C, add 9.45g (0.105 moles) of the compound shown in formula a-2 into the reaction flask, and dropwise add 35.8g (0.1 moles) of the compound shown in formula g-1 and 160 A solution consisting of one milliliter of N-methylpyrrolidone was added within 30 minutes and reacted at room temperature for 10 hours. After TLC detected that the reaction was complete, an appropriate amount of water was added, and the resulting mixed solution was adjusted to pH 10.0 to 11.0 with 2 mol / liter aqueous sodium hydroxide solution, and a large amount of solid was precipitated, filtered by suction, washed, and dried to obtain a crude off-white solid. Recrystallize with isopropanol / acetone (isopropanol / acetone=2:1 (v / v)), and purify to obtain a white solid powder, which is 25.6 g of the compound shown in formula I-2, with a yield of 62.1% and a purity of 99.4 %.
[0094] L...
Embodiment 3
[0095] Embodiment 3: the preparation of the compound shown in formula I-3
[0096] The preparation method of the compound shown in formula I-3 is the same as that of Example 2, the only difference being that the reaction raw materials are different. Yield 55.7%, purity 98.6%.
[0097] LCMS: 413 (M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com